Acids Bases Salts Class 10 Science Important Questions
Please refer to Acids Bases Salts Class 10 Science Important Questions with answers below. These solved questions for Chapter 2 Acids Bases Salts in NCERT Book for Class 10 Science have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 10 Science for all chapters in your textbooks.
Important Questions Class 10 Science Chapter 2 Acids Bases Salts
All Acids Bases Salts Class 10 Science Important Questions provided below have been prepared by expert teachers of Standard 10 Science. Please learn them and let us know if you have any questions.
Very Short Answer Type Questions
Question: The pH of soil A is 7.5 while that of soil B is 4.5. Which of the two soils should be treated with chalk and why?
Answer: Chalk or CaCO3 is basic in nature. As the soil B having pH 4.5 is acidic, it should be treated with powdered chalk to reduce its acidity by increasing its pH. Soil A is slightly alkaline.
Question: Two solutions X and Y are tested with universal indicator. Solution X turns orange whereas solution Y turns red. Which of these is a stronger acid?
Answer: The universal indicator produces red colour for the strongest acid and orange colour for slightly weaker acid. As solution X turns orange whereas solution Y turns red, Y is a stronger acid than X.
Question: What types of ions are formed: (a) when an acid is dissolved in water (b) when a base is dissolved in water ?
Answer: When an acid is dissolved in water, it forms hydrogen or H+ ions whereas it forms hydroxide or OH- ions when a base is dissolved in water.
Question: Name the acid along with itschemical formula present in ant sting.
Answer: The acid present in ant sting is methanoic acid (formic acid). The chemical formula is HCOOH.
Explanation: When an ant stings, it leaves formic acid (Methanoic acid) which causes pain and irritation. To get relief from the sting, mild base like baking soda on the stung area gives relief.
Question: A white chemical compound becomes hard on mixing proper quantity of water. It is also used in surgery to maintain joints in a fixed position. Name the chemical compound.
Answer: The white compound that is used in surgery to maintain joints in a fixed position and also becomes hard on mixing water is called Plaster of Paris or calcium sulphate hemihydrates. It has a property of setting into a hard mass on wetting with water which is due to its hydration to form crystals of gypsum which set to form a hard solid mass.
CaSO4. 1/2H2O + 1 1/2H2O → CaSO4.2H2O
Question: When electricity is passed through a common salt solution, sodium hydroxide is produced along with the liberation of two gases “X” and “Y”. “X” burns with a pop sound whereas “Y” is used for disinfecting drinking water. Identify X and Y.
Answer: The reaction is chlor-alkali process. As X burns with a pop sound, X is hydrogen gas. Y is chlorine gas which is used for disinfecting drinking water.
Short Answer Type Questions
Question: (A) Draw a labelled diagram to show the preparation of hydrogen chloride gas in laboratory.
(B) Test the gas evolved first with dry and then with wet litmus paper. In which
of the two cases, does the litmus paper show change in colour?
(C) State the reason of exhibiting acidic character by dry HCl gas/HCl solution.
Answer: (A)

(B) Add concentrated sulphuric acid to the test tube containing 1 g of solid sodium chloride.
Hydrogen chloride gas will be formed as per the given equation :
H2SO4(l) + 2NaCl(s) → NaHSO4(l)+2HCl(g)
Conc. Sodium Sodium Hydrogen
sulphuric chloride sulphate chloride
acid gas
Test gas with wet litmus paper: Test the gas with wet blue litmus paper, its colour changes to red Now test with wet red litmus paper, There will be no change in colour.
(C) Dry HCl gas does not exhibit the acidic character. HCl solution exhibit acidic character. HCl acts as an acid only in the presence of water as moist litmus paper has water in which HCl gas dissolves and then it dissociates/ ionises into (H+)(aq) hydrogen ions and chloride ions.
The H+(aq) are responsible for the acidic character.
HCl + H2O → H2O+ + Cl–
Question: 2 g of ferrous sulphate crystals are heated in a dry boiling tube:
(A) List any two observations.
(B) Name the type of chemical reaction taking place.
(C) Write the chemical equation for the reaction.
(D) Name the products obtained.
Answer: 2 g of ferrous sulphate crystals are heated in a dry boiling tube.
(A) We observe that the green coloured ferrous sulphate crystals on heating changes to reddish brown ferric oxide. Also it gives out a characteristic smell of burning sulphur.
(B) It is thermal decomposition of ferrous sulphate.
(C) The chemical equation for the reaction is
2FeSO4Δ→ Fe2O3 + SO2 + SO3
(D) Products obtained are Ferric oxide, Sulphur dioxide and sulphur trioxide.
Question: Answer the following:
(A) How is tooth decay related to pH? How can it be prevented?
(B) What is the change in colour of pH paper dipped in a solution having a pH = 13?
Answer: (A) When we eat food containing sugar, then the bacteria present in our mouth break down the sugar to form acids such as lactic acid. This acid lowers the pH in the mouth making it acidic. Tooth decay starts when the pH of acid formed in the mouth falls below 5.5. This is because then the acid becomes strong enough to attack the enamel of our teeth and corrode it. This sets in tooth decay.
The best way to prevent tooth decay is to clean the mouth thoroughly after eating food by rinsing it with lots of clean water.
Many tooth pastes contain bases to neutralise the mouth acid. The pH of tooth paste is about 8.0. Therefore, using the tooth paste, which is generally basic, for cleaning the tooth can neutralise the excess acid in mouth and prevent tooth decay.
(B) The solution with highest pH (13) will have minimum hydrogen ion concentration i.e. alkaline solution and so will change colour of pH paper to light violet colour.
Question: List the important products of the Chlor-alkali process. Write one important use of each.
Answer: When electricity is passed through brine (an aqueous solution of sodium chloride), it decomposes
2NaCl(aq) + 2H2O(l) Electric → Current 2NaOH(aq) + H2(g) + Cl2(g)
The process of breakdown of brine solution is called Chlor-alkali process because of products ‘chlor‘ for chlorine and ‘alkali‘ for sodium hydroxide.
Chlorine gas is formed at the cathode, hydrogen gas at the cathode whereas sodium hydroxide solution is formed near the cathode.
Use of Products formed:
(1) Uses of NaOH (sodium hydroxide): It is used for:
• de-greasing metals
• Manufacture of soaps, paper, a large number of chemicals and artificial fibres
(Any one)
(2) Uses of Chlorine — It is used in:
• Water treatment, swimming pool
• Preparation of bleaching powder, PVC, disinfectants, CFC’s Pesticides etc.
(3) Uses of Hydrogen: It is used:
• Inmanufacture of ammonia for fertilizers as rocket fuel (Any one)
Question: How is washing soda prepared from sodium carbonate? Give its chemical equation.
State the type of this salt. Name the type of hardness of water which can be removed by it?
Answer: Preparation of washing soda from sodium carbonate Anyhydrous sodium carbonate is dissolved in water i.e.
soda Na2CO3.10H2O
Na2CO3 + 10H2O → Na2CO3.10H2O
Sodium Carborate Washing soda
It is a basic salt.
Permanent hardness can be removed by washing soda.
Question: What do you understand by olfactory indicators?
Answer: The substances whose smell changes in acidic or basic solutions are called olfactory indicators. An olfactory indicator usually works on the principle that when an acid or base is added to it, it loses its characteristic smell. Onion and vanilla extract are examples of olfactory indicators.
Question: A compound ‘X’ of sodium is used as an antacid and it decomposes on strong heating.
(A) Name the compound ‘X’ and give its chemical formula.
(B) Write a balanced chemical equation to represent the decomposition of ‘X’.
(C) Give one use of compound ‘X’ besides an antacid.
OR
You are provided with 90 mL of distilled water and 10 mL of concentrated sulphuric acid to prepare dilute sulphuric acid.
(A) What is the correct way of preparing dilute sulphuric acid? Give reason.
(B) How will the concentration of H3O + ions change on dilution?
Answer: (A) Sodium bicarbonate/Sodium hydrogen carbonate/ baking soda and its formula is NaHCO3.
(B) 2NaHCO3 Heat → Na2CO3 + CO2 + H2O
(C) It is used in fire extinguisher and for baking. (any one)
OR
(A) Add 10 mL of concentrated sulphuric acid slowly to 90 mL of water with constant stirring.
Dilution of acid is a highly exothermic process. If water is added to concentrated sulphuric acid, heat generated causes the mixture to splash leading to burns and the glass container can break.
(B) Decreases per unit volume.
Question: Explain the action of dilute hydrochloric acid on the following with balanced chemical equations and observations for the reactions:
(A) Magnesium ribbon
(B) Sodium hydroxide
(C) Crushed egg shells
Answer: Action of dilute hydrochloric acid on the following:
(A) Magnesium ribbon: When magnesium metal reacts with an aqueous solution of hydrochloric acid, we will get dissolved magnesium chloride and hydrogen gas.
Mg(s) + 2HCl(aq) → H2(g) + MgCl2(aq)
(B) Sodium hydroxide: There is no noticeable change in the appearance of the solution, it remains colorless like water.
HCl + NaOH → NaCl + H2O + heat
(C) Crushed egg shells: Egg shells contain calcium carbonate CaCO3. So when we react CaCO3 with HCl, the egg shells completely dissolve and there will be brisk effervescence due to CO2.
CaCO3 + HCl → CaCl2 + H2O + CO2
Question: What is the effect of following on acetic acid?
(A) litmus test.
(B) addition of a pinch of baking soda.
Answer: (A) Effect on litmus: Blue litmus turns red which proves that acetic acid is acidic in nature.
(B) Reaction with baking soda: A gas is evolved which turns lime water milky.
The gas produced is carbon dioxide due to the action of CH3COOH on NaHCO3, which turns lime water milky
CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Question: Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B. In which test tube will the fizzing occur more vigorously and why?
Answer: Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A, while acetic acid (CH3COOH) is added to test tube B.
In both cases, hydrogen gas is evolved. When metal reacts with acid it forms salt and hydrogen gas.
Metal + Acid → Salt + Hydrogen gas
Fizzing will occur more vigorously in test tube A containing hydrochloric acid. This is because hydrochloric acid is stronger acid than acetic acid and reaction between magnesium ribbon and HCl is faster in test tube A than the reaction between Magnesium and acetic acid in test tube B.
Long Answer Type Question:
Question: A cloth strip dipped in onion juice is used for testing a liquid ‘X‘. The liquid ‘X‘ changes its odour. Which type of an indicator is onion juice? The liquid ‘X‘ turns blue litmus red. List the observations the liquid ‘X‘ will show on reacting with the following:
(A) Zinc granules
(B) Solid sodium carbonate
Write the chemical equations for the reactions involved.
Answer: Onion juice is an olfactory indicator. Those substances whose smell or odour changes in acidic or basic solution are called olfactory indicator. The liquid ‘X‘ turns blue litmus red. It is an acidic liquid. An acidic solution does not destroy the smell of onions whereas when a basic solution like sodium hydroxide solution is added to a cloth strip treated with onion juice, the onion smell can not be detected. That is why onion juice can be used a test for acids and bases. Onion has mild sulphuric acid and when we cut it, it dissolves in air forming acid and on reaching our eyes causes burning sensation and our tear glands flush out the eyes.
Hence, Liquid ‘X‘ is dilute sulphuric acid (H2SO4) Observations when liquid ‘x‘ reacts with
(A) Zinc granules: When zinc granules are added to liquid ‘x‘ (H2SO4) in a test tube a brisk reaction takes place with evolution of hydrogen gas.
The test tube becomes hot.
When a burning match stick is brought near a gas filled bubble, the gas present in the bubble burns with a popsound.

(B) Solid sodium carbonate: When liquid ‘X‘ reacts with solid sodium carbonate, a salt, carbon dioxide and water are formed.
Brisk effervescence of carbon dioxide gas is produced.
When CO2 is passed through lime water, lime water turns milky.

(C) Chemical equations
(i) Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Zinc Dilute zinc Hydrogen
(metal) Sulphuric (sulphate gas
acid salt)
(ii) Na2CO3(s) + H2SO4(aq) → Na2SO4(aq)+ CO2(g) + H2O(l)
Sodium Sulphuric Sodium Carbon water
carbonate acid sulphate dioxide
Question: Baking powder is used for baking a cake. If your mother uses baking soda instead of baking powder in cake:
(A) How will it affect the taste of the cake and why?
(B) How can baking soda be converted into baking powder?
(C) What is the role of tartaric acid added to baking soda?
Answer: (A) Baking soda is sodium hydrogen carbonate which decomposes to sodium carbonate, water and carbon dioxide on heating.
Baking powder is a mixture of sodium hydrogen carbonate with tartaric acid, which readily reacts with sodium carbonate and neutralises it.
(B) Baking soda can be converted into baking powder by the addition of an appropriate amount of tartaric acid to it.
(C) The role of tartaric acid is to neutralise sodium carbonate and the cake will not taste bitter.
Question: Varun took a sample A and added dilute hydrochloric acid to it. A colorless, odorless gas X was evolved which turned lime water milky.
(A) Identify sample A and the gas X evolved.
(B) Write a chemical equation to explain the reaction between sample A and hydrochloric acid.
(C) Why does the gas X turn lime water milky?
Answer: (A) We know that acid reacts with a metal carbonate or metal hydrogencarbonate to form a salt, carbon dioxide gas and water. This carbon dioxide gas when passed through lime water turns it milky in appearance. With this statement, we can say that sample A is either metal carbonate or metal hydrogencarbonate and gas X is carbon dioxide gas.
(B) Let us now see the possible reactions of dilute hydrochloric acid with a metal carbonate and metal hydrogencarbonate:

Question: Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.
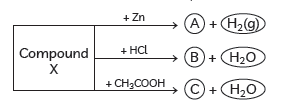
Answer: X must be a compound which forms water with acids. It means it must be a base which reacts with acids to form salt and water. This base also reacts with zinc metal and releases hydrogen gas. So, it must be NaOH (sodium hydroxide).
2NaOH + Zn → Na2ZnO2 + H2(g)
Sodium zincate
(A)
NaOH + HCl → NaCl + H2O
Sodium chloride
(B)
NaOH + CH3COOH → CH3COONa + H2O
Sodium acetate
(C)
X—NaOH (Sodium hydroxide)
A—Na2ZnO2 (Sodium zincate)
B—NaCl (Sodium chloride)
C—CH3COONa (Sodium acetate)
Question: A white powder is used by doctors to support fractured bones.
(A) Write the name and chemical formula of the powder.
(B) How is this prepared?
(C) When this white powder is mixed with water, a hard solid mass is obtained. Write a balanced chemical equation for the change.
(D) Give one more use of this powder.
Answer: (A) The white powder used by doctors to support fractured bones is called plaster of Paris. It is chemically called calcium sulphate hemihydrates or calcium sulphate half-hydrate. The chemical formula of plaster of Paris is
CaSO4. 1/2 H2O.
(B) Plaster of Paris is prepared from gypsum. Chemically, gypsum is calcium sulphatedihydrate, CaSO4.2H2O. Plaster of Paris is prepared by heating gypsum (CaSO4.2H2O) to a temperature of 100°C in kiln. When gypsum is heated to a temperature of 100°C, it loses three-fourths of its water of crystallisation and forms plaster of Paris:

(D) Apart from setting fractured bones, plaster of Paris is used in chemistry laboratories for sealing air-gaps in apparatus where air- tight arrangement is required.
Question: (A) Why does acidic solution conduct electricity?
(B) Can basic solution conduct electricity?
(C) Can separation of H+ ions in acids take place when HCl is added to a non-aqueous solution?
(D) While diluting an acid, why is it recommended that the acid should be addd to water and not water to the acid?
Answer: (A) The aqueous solution of an acid conducts electricity due to the presence of charged particles called ions in it. For example, when hydrochloric acid (HCl) is dissolved in water, its solution contains hydrogen ions, H+(aq) and chloride ions, Cl–(aq).
These ions can carry electric current. So, due to the presence of H+ (aq) ions and Cl–(aq) ions, a solution of hydrochloric acid conducts electricity.
(B) When a base is dissolved in water, it splits up into ions. Due to the presence of ions, the solutions of bases also conduct electricity.
(C) Noseparation of H+ ions does not take place when HCl is added to a non-aqueous solution. An acid always ionizes on dissolving in water to produce hydrogen ions.
(D) A concentrated acid is always diluted by adding water to it. The process of mixing water to a concentrated acid is a highly exothermic process. In this process, a large amount of heat is evolved.
• When concentrated acid is added slowly to excess water, the heat is evolved gradually and easily absorbed by the large amount of water.
• If water is added to excess concen- trated acid, a large amount of heat is evolved suddenly. This heat uses some of the water to steam explosively. This
results in a splash of acid on our body and causes acid burns.
Question: On adding a few drops of universal indicator in three colourless solutions X, Y and Z taken separately in three test tubes, a student observed the changes in colour as green in X, red in Y and blue in Z.
(A) Arrange X, Y and Z in increasing order of their pH values.
(B) Which one of the three, X, Y and Z, will change the colour of phenolphthalein ?
Why ?
OR
State the observation and inference made by a student when he brings (i) a wet blue
litmus paper and (ii) a wet red litmus paper in contact with the gas liberated during thermal decomposition of ferrous sulphate.
Answer: (A) Y, X, Z
(B) Z, because it is basic in nature and the bases turn phenolphthalein pink.
OR
(i) Observation: The moist blue litmus paper will turn red.
Inference: The gas liberated is acidic in nature.
(ii) Observation: Wet red litmus paper will remain red.
Inference: The gas liberated is acidic in nature.
Question: Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1,11, 7 and 9 respectively. Which solution is:
(A) Neutral (B) Strongly alkaline
(C) Strongly acidic (D) Weakly acidic
(E) Weakly alkaline
Arrange the pH in increasing order of hydrogen ion concentration.
Answer: Given pH for the solutions are
A = 4, B = 1, C = 11, D = 7, E = 9.
Hydrogen ions concentration increases with decrease in pH value and thus strength of acid increases with decrease in pH value from 7 to 0.
On the other hand hydroxide ions concentration decreases with increase in pH value and thus strength of acid increases with increase in pH value from 7 to 14.
While neutral solution has pH value = 7.
Therefore,
(A) Solution D is neutral having pH value equal to 7.
(B) Solution C is strongly alkaline as its pH value is equal to 11
(C) Solution B is strongly acidic as its pH value is equal to 1
(D) Solution A is weakly acidic as its pH value is equal to 4
(E) Solution E is weakly alkaline as its pH value is equal to 9
Hydrogen ion concentration of the given solutions will be
A = 10–4 M, B = 10–1M, C = 10–11M, D = 10–7M, E = 10–9M.
Hence arrangement of given pH value in increasing order of hydrogen ion concentration:
C (11) < E (9) < D (7) < A (4) < B(1)
Question: Identify the acid and the base from which sodium chloride is obtained. Which type of salt is it? When is it called rock salt? How is rock salt formed?
Answer: Sodium chloride or NaCl is obtained by the reaction between sodium hydroxide solution and hydrochloric acid.
NaOH + HCl → NaCl + H2O
Sodium chloride is a neutral salt as it is formed by the reaction between a strong acid and a strong base.
It is called rocksalt when found in the form of large crystals which are often brown due to impurities.
Rock salt is formed by evaporation of seawater, as seawater contains many dissolved salts, including sodium chloride.
Question:(A) A student prepared solutions of (i) an acid and (ii) a base in two separate beakers. She forgot to label the solutions and litmus paper is not available in the laboratory.
Since, both the solutions are colourless, how will she distinguish between the two?
(B) What is an olfactory indicator? Name two olfactory indicators. What is the effect of adding sodium hydroxide solution to these olfactory indicators?
Answer: (A) Other than litmus paper, pH indicators can also be used to distinguish between an acidic and an alkaline solution. Some common pH indicators are methyl orange, phenolphthalein. They show characteristic colour change in acidic and alkaline medium.
Some natural indicators such as turmeric, red cabbage juice can also used to identify acidic and alkaline behavior of solutions.
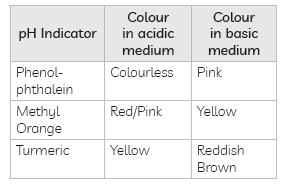
(B) Olfactory Indicators: Substances which change their smell when mixed with acid or base are known as olfactory indicators.
Two olfactory indicators are onion and vanilla.
When sodium hydroxide solution is added to an olfactory indicator it loses its characteristic smell.
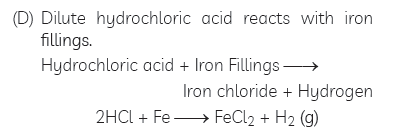
Question: Write word equations and then balanced equations for the reactions taking place when:
(A) dilute sulphuric acid reacts with zinc granules.
(B) dilute hydrochloric acid reacts with magnesium ribbon.
(C) dilute sulphuric acid reacts with aluminium powder.
(D) dilute hydrochloric acid reacts with iron fillings.
(E) sodium hydroxide reacts with hydrochloric acid.
Answer:

Question: Answer the following:
(A) Mention the pH range within which our body works. Explain how antacids give relief from acidity. Write the name of one such antacid.
(B) Fresh milk has a pH of 6. How will the pH change as it turns to curd? Explain your Answer:
(C) A milkman adds very small amount of baking soda to fresh milk. Why does this milk take longer time to set as curd?
(D) Mention the nature of tooth pastes. How do they prevent tooth decay?
Answer: (A) Our body works roughly between 7.3 to 7.5 pH range.
Acidity means excess of acid level in stomach. Antacid contains basic salt and are alkaline. So when we take antacid it reacts with excess acid of stomach and neutralizes it.(neutralization reaction takes place between acid and base).
Milk of Magnesia is a commonly used antacid.
(B) Fresh milk has a pH of 6. When fresh milk turns to curd, it produce lactic acid (acidic in nature) which alters the pH of fresh milk i.e. pH will decrease. This is because curd is more acidic in nature than milk as it contains lactic acid. More acidic is a substance, lesser is its pH.
(C) pH of fresh milk is around 6. When the milk sets to curd, the pH decreases i.e. it becomes more acidic. The presence of alkali does not allow it to become more acidic easily. Hence, it will take long time to set as curd.
(D) Tooth decay is caused at acidic pH whichis produced by bacteria in our mouth. Our tooth paste is slightly basic or alkaline in nature .It remove excess of acid from mouth when we brush. In this way it maintains the pH of mouth as neutral and decreases the chance of decaying.

