Acids Bases Salts Class 10 Science Important Questions
Please refer to Acids Bases Salts Class 10 Science Important Questions with answers below. These solved questions for Chapter 2 Acids Bases Salts in NCERT Book for Class 10 Science have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 10 Science for all chapters in your textbooks.
Important Questions Class 10 Science Chapter 2 Acids Bases Salts
All Acids Bases Salts Class 10 Science Important Questions provided below have been prepared by expert teachers of Standard 10 Science. Please learn them and let us know if you have any questions.
Question: Why does 1 M HCl solution have a higher concentration of H+ ions than 1 M CH3COOH solution?
Answer: 1 M HCl solution have a higher concentration of H+ ions than 1 M CH3COOH solution because HCl is a strong acid than CH3COOH. Hence the H in HCl is able to dissociate very quickly from its formula unit mass and gets liberated in the form of H+very quickly leading to a rapid increase in the H+ concentration but CH3COOH is a weak acid and the H present in it is not able to dissociate quickly and completely from its formula unit mass in the form of H+ ions which leads to a slow increase in the H+ ion concentration and a low concentration of H+ ions in the solution.
Question: What is common name of the compound CaOCl2?
Answer: The common name of the compound CaOCl2 is bleaching powder. It is used as a bleaching agent for bleaching cotton, linen, wood pulp, clothes, as an oxidizing agent in chemical industry and for disinfecting drinking water.
Question: Name the substance which on treatment with chlorine yields bleaching powder.
Answer: Dry slaked lime [Ca(OH)2] on treatment with chlorine yields bleaching powder.
The chemical equation representing its formation is: –
Ca(OH)2 + Cl2→ CaOCl2 + H2O
Question: Name the sodium compound which is used for softening hard water.
Answer: The sodium compound which is used for softening hard water is called sodium carbonate which is most commonly known as washing soda. It is prepared by heating baking soda and then recrystallizing the product so obtained to get washing soda.
Question: Which one of these has a higher concentration of H+ ions? 1 M HCl or 1 M CH3COOH.
Answer: 1 M HCl has a higher concentration of H+ ions than 1 M CH3COOH because HCl is a strong acid then CH3COOH hence 1M HCL produces more amount of H+ ions than that produced by 1 M CH3COOH.
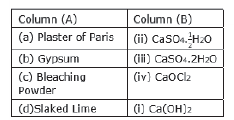
Question: Math the important chemicals given in Column (A) with the chemical
formulae given in Column
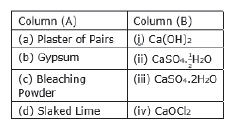
Answer:
Question: A student dropped few pieces of marble in dilute HCl contained in a test
tube. The evolved gas was passed through lime water.
(a) What change would be observed in lime water?
(b) Write balanced chemical equation for the above change.
Answer: a. Marble is chemically composed of calcium carbonate (CaCO3). According to the question, the student dropped few pieces of marble in dilute HCl contained in a test tube. We know that acids react with calcium carbonate to form or give the corresponding salt, carbon dioxide and water. Hence, when we pass the carbon dioxide gas through lime water a white precipitate would be observed to form in the test tube containing the lime water.
b. The balanced chemical equation for the above change when we pass the carbon dioxide gas through lime water is:
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
Question: Name the acid present in the following:
(a) Tomato
(b) Vinegar
(c) Tamarind
Answer: a. The acid present in tomato is oxalic acid.
b. The acid present in vinegar is acetic acid.
c. The acid present in tamarind is tartaric acid.
Question: (a) What is an alkali? Give an example.
(b) Why do HCl, HNO3, etc. show acidic characters in aqueous solutions while solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer: a. The bases which are soluble in water are called alkali. Sodium hydroxide is a base which is soluble in water and hence it is an alkali.
b. A compound is said to be acidic in character if it is able to generate H+ ions in aqueous solutions. HCl, HNO3, etc. show acidic characters in aqueous solutions because they are able to generate H+ ions in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character because they are not able to generate H+ ions in aqueous solutions.
Question: Fill in the missing data in the following table:

Answer:

Question: A white coloured powder is used by doctors for supporting fractured bones.
(a) Write chemical name and formula of the powder.
(b) When this white powder is mixed with water a hard-solid mass is obtained.
Write balanced chemical equation for the change.
Answer: a. The chemical name and formula of the white coloured powder which is used by doctors for supporting fractured bones is: –
Chemical name – Calcium sulphate hemihydrate
Formula – CaSO4 1/2 H2O
b. When this white powder is mixed with water a hard-solid mass is obtained. The balanced chemical equation for the change is: –
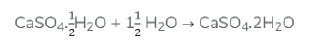
Question: A gas ‘X’ reacts with lime water and forms a compound ‘Y’ which is used as a bleaching agent in chemical industry. Identify ‘X’ and ‘Y’. Give the chemical equation of the reactions involved.
Answer: Chlorine gas reacts with lime water to form bleaching powder which is used as a bleaching agent in chemical industry.
Hence, the species X is Chlorine gas and the species Y is Bleaching powder with formula -CaOCl2.
The chemical equation of the reactions involved is: –
Ca(OH)2 + Cl2→ CaOCl2 + H2O
Question: What is the colour of FeSO4.7H2O crystals? How does this colour change upon heating? Give balanced chemical equation for the changes.
Answer: The colour of FeSO4.7H2O crystals is light green. Upon heating the crystals of FeSO4 7H2O losses water of crystallization and becomes white in colour, Balanced chemical equation for the change is: –
2FeSO4→Δ Fe2O3 + SO2 + SO3
Question: A compound which is prepared from gypsum has the property of hardening when mixed with proper quantity of water.
(a) Identify the compound.
(b) Write the chemical equation for its preparation.
(c) Mention one important use of this compound.
Answer: a. The compound which is prepared from gypsum which has the property of
hardening when mixed with proper quantity of water is called plaster of Paris having the formula – CaSO4.1/2 H2O.
b. The chemical equation for the preparation of plaster of Paris is: –
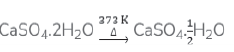
Plaster of Paris is prepared by heating gypsum at 373K.
c. One of the important use of plaster of Paris is that it is used by doctors as a plaster for supporting fractured bones in the right position
Question: A. Explain the following chemical properties of acids with the help of balanced chemical equations only.
(i) When an acid reacts with a metal carbonate
(ii) When an acid reacts with a metal bicarbonate
(iii) When an acid reacts with a metal oxide
Answer: i. When hydrochloric acid reacts with sodium carbonate the following reactions takes place: –
Na2CO3(s) + 2HCl (aq) → 2NaCl(s) + H2O (l) + CO2 (g)
Hence, when an acid reacts with a metal carbonate the corresponding salt is formed
along with the evolution of carbon dioxide gas and the formation of water.
ii. When hydrochloric acid reacts with sodium hydrogen carbonate the following
reactions takes place: –
NaHCO3(s) + HCl (aq) → NaCl(s) + H2O (l) + CO2 (g)
Hence, when an acid reacts with a metal hydrogen carbonate the corresponding salt is formed along with the evolution of carbon dioxide gas and the formation of water.
iii. When dilute hydrochloric acid reacts with copper oxide the following reactions takes place: –
CuO(s) + 2HCl (aq) → 2CuCl2(s) + H2O (l)
Hence, when an acid reacts with a metal oxide the corresponding salt is formed along with the formation of water.
This reaction could be seen as similar to the reaction between an acid and a base. So, metallic oxides are treated as basic in nature.
Question: B You are given three solutions A, B and C with pH values 2, 10 and 13
respectively. Write which solution has more hydrogen ions concentration among
the three and state the nature ‘acidic or basic’ of each solution.
Answer: b. According to the theory on a pH scale, a more acidic substance is assigned
a lower value. Normally substances with pH less than 7 are considered as acidic and
those with pH greater than 7 are considered as basic with acidic strength increasing as
we move towards 1 from 7 and the basic strength increases as we move toward 14 from
7. The more the acidic a substance is the more the hydrogen ions concentration will be.
Hence, in the given solutions, solution A has more hydrogen ions concentration as it is
having the least pH value out of the three.
The nature of each solution is: –
Solution A – acidic
Solution B – basic
Solution C – basic
Question: A. A metal compound ‘X’ reacts with dil.H2SO4 to produce effervescence.
The gas evolved extinguishes a burning candle. If one of the compounds formed
is calcium sulphate, then what is ‘X’ and the gas evolved? Also, write a balanced
chemical equation for the reaction which occurred.
Answer: Given that the metal compound ‘X’ reacts with dil.H2SO4 to produce
effervescence and the gas evolved extinguishes a burning candle. Now, since the gas
evolved extinguishes the candle it must be carbon dioxide. It is also given that one of the compounds formed is calcium sulphate, hence the metal compound X is calcium carbonate.
Hence, the reaction between calcium carbonate and dil.H2SO4 is as follows: –
CaCO3 + H2SO4→ CaSO4 + CO2 + H2O
Question: B. (i) Name one antacid. How does it help to relieve indigestion in stomach?
(ii) A farmer treats the soil with quicklime or calcium carbonate. What is the nature of soil? Why does the farmer treat the soil with quicklime?
Answer: i. Name of one antacid is Magnesium Hydroxide (Milk of magnesia).
During indigestion, our stomach produces too much acid and this causes irritation.
Hence, when we take a base it neutralises the effect of the excess acid and relieves us from the irritation and indigestion.
ii. Quicklime or calcium carbonate is basic in nature. Hence, the nature of the soil must be acidic.
Plants grow well in the pH range of 6 – 8. So, when the soil becomes acidic in nature
the farmers add bases like quicklime or calcium carbonate to neutralise the excess acid and maintain the pH.
Question: (a) Identify the acid and the base whose combination forms the common salt that you use in your food. Write its formula and chemical name of this slat. Name the source from where it is obtained.
(b) What is rock salt? Mention its colour and the reason due to which it has this colour.
(c) What happens when electricity is passed through brine? Write the chemical equation for it.
Answer: a. The common salt that we use in your food is obtained by the combination of base – sodium hydroxide (NaOH) and the acid – hydrochloric acid (HCl).
The formula for the common salt that we use in your food is NaCl and its name is sodium chloride.
It is obtained from seawater.
b. When the seas of bygone ages dried-up beds of rock salt were being formed. Rock salts are the deposits of solid salts.
The colour of rock salt is brown. It is brown in colour due to the impurities present in it.
c. When electricity is passed through brine it decomposes to form sodium hydroxide along with the evolution of chlorine gas at anode and hydrogen gas at cathode. Sodium hydroxide solution is also formed near the cathode.
The chemical equation for the above decomposition is: –
2NaCl (aq) +2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Question: (a) Identify the compound of calcium which is yellowish white powder and is used for disinfecting drinking water. Write its chemical name and formula. How is it manufactured? Write the chemical equation for the reaction involved. Also, list two other uses of the compound.
(b) Write the balanced chemical equation of chlor-alkali process.
Answer: (a) The compound of calcium which is a yellowish white powder and is used for disinfecting drinking water is CaOCl2 commonly known as bleaching powder.
Its chemical name is – Calcium oxychloride Formula – CaOCl2 Formation of bleaching powder.
When chlorine gas is allowed to pass through the slaked lime solution leads to the formation of bleaching powder. The chemical equation for the reaction involved is: –
Ca(OH)2 + Cl2→ CaOCl2 + H2O
Two other uses of the compound are:-
1. It is used as an oxidising agent in many chemical industries.
2. It is used for bleaching cotton and linen in the textile industry, wood pulp bleaching and for bleaching washed clothes in the laundry.
(b) The balanced chemical equation of chlor-alkali process is: –
2NaCl (aq) +2H2O (l) → 2NaOH (aq) + Cl2 (g) + H2 (g)
Question: In the following schematic diagram for the preparation of hydrogen gas as shown in the given figure, what would happen if the following changes are made?

(a) In place of zinc granules, same amount of zinc dust is taken in the test tube.
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
(c) In place of zinc, copper turnings are taken.
(d) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
Answer: a. If in the place of zinc granules, same amount of zinc dust is taken in the test tube then the rate of the reaction will increase and the reaction will take place more quickly. This is because the surface area per unit volume of powder is more than that of the zinc granules and hence the powder will provide more surface area for the reaction to take place.
b. If in place of dilute sulphuric acid, we use dilute hydrochloric acid then the reaction will take place as usual because in both the cases we are mixing a strong acid with a metal.
c. If in place of zinc, copper turnings are taken then also the reaction will proceed in a similar way and would lead to the evolution of hydrogen gas as here we are only replacing one metal with another metal in the reactant side of the reaction. In this case, also we are mixing a strong acid with a metal.
d. If sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated then the metal will react with the base to form the corresponding salt and will evolve hydrogen gas as per the following reaction:-
Zn + 2NaOH → Na2ZnO2 + H2
Question: (a) Crystals of a substance changed their colour on heating in a closed test tube but regained it after sometime when they were allowed to cool down. Name the substance and write its formula and explain the phenomenon involved.
(b) Name the compound whose one formula unit is associated with 10 water molecules. How is it prepared? Give equations of related reactions. Give two uses of the compound.
Answer: a) Crystals of a substance which changed their colour on heating in a closed test tube but regained it after sometime when they were allowed to cool down are copper sulphate or more specifically hydrated copper sulphate.
Its formula is – CuSO4.5H2O The phenomena involved is due to the presence of molecules of water of crystallization in the molecule. Water of crystallization is the fixed number of water molecules that are present in one formula unit of a salt. When we heat the crystals of hydrated copper sulphate this water is removed and the salt turns white. But when it is allowed to cool down in open air then it regains its lost water of crystallization and hence regains its colour also. b) The compound whose one formula unit is associated with 10 water molecules is sodium carbonate (Na2CO3. 10H2O) commonly known as washing soda.
It is prepared by heating baking soda and then recrystallizing the product so obtained.
The equations related to the above reaction is: –
2NaHCO3→Δ Na2CO3 + H2O + CO2
Na2CO3 + 10H2O → Na2CO3. 10H2O
Two uses of washing soda are: –
1. It is used in glass, soap and paper industries
2. It is used for removing permanent hardness of water.
Question: Hritik tested rainwater and found a pH of 5-6 due to presence of H+ ions formed by reaction of rainwater with carbon dioxide. When pH of the rainwater drops below 5-6, then it is called acid rain. Oxides of nitrogen and sulphur are acidic in nature. Burning of fossil fuels such as coal and oil in power stations and furnace produce oxides of nitrogen and sulphur. He told all of his classmates living nearby to use bicycle instead of school bus so as to reduce air pollution and prevent acid rain.
(a) Which acid is formed by sulphur dioxide in presence of air?
(b) Which acid is formed by nitrogen dioxide in presence of air?
(c) Why is acid rain harmful for agriculture, trees and plants?
(d) How can we prevent from acid rain?
(e) What values are possessed by Hiritik?
Answer: a. Sulfuric acid, sulphurous acids are formed by sulphur dioxide in presence of air.
b. Nitric acid is formed by nitrogen dioxide in presence of air.
c. Plants, agricultural crops and trees require an optimum pH for their survival. Due to acid rain, the pH balance of the soil is disturbed and the soil becomes more acidic. This acidic nature of the soil is very much harmful to the healthy growth of plants, agricultural crops and trees. In this way, acid rain is harmful to agriculture, trees and plants.
d. We can prevent acid rain by controlling pollution, by controlling the rate of consumption and burning of fossil fuels such as coal and petroleum oil, by controlling automobile combustion, reducing the burning of wastes, filtering out particulate matters emitted from the industries, using cleaner fuels like CNG in the automobiles and start using renewable sources of energy like solar energy, wind energy, tidal energy etc for electricity generation.
e. Hritik possesses the value of social responsibility. He is very much concerned for his environment and the health of the society as a whole.
