Atoms And Molecules Class 9 Science Important Questions
Please refer to Atoms And Molecules Class 9 Science Important Questions with answers below. These solved questions for Chapter 3 Atoms And Molecules in NCERT Book for Class 9 Science have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 9 Science for all chapters in your textbooks.
Important Questions Class 9 Science Chapter 3 Atoms And Molecules
All Atoms And Molecules Class 9 Science Important Questions provided below have been prepared by expert teachers of Standard 9 Science. Please learn them and let us know if you have any questions.
Short Answer Type Questions
Question: 8.4 g of sodium bicarbonate on reaction with 20 g of acetic acid liberated 4.4 g of carbon dioxide gas into the atmosphere. What is the mass of the residue left?
Answer: Total mass of reactants = 8.4 g + 20 g = 28.4 g
Total mass of reactants = Total mass of products
28.4 = 4.4 + x (mass of residue left)
28.4 – 4.4 = x ⇒ x = 24 g
Question: How many particles are represented by 0.25 mole of an element?
Answer: mole of an element contains 6.022 × 1023 particles
0.25 mole of an element contains = 6.022 × 1023 × 0.25
= 1.5 × 1023 particles
Question: Why does the atomic mass of an element not represent the actual mass of its atom?
Answer: Atomic mass of an element is the mass of its atom on the atomic scale, on the other hand, the actual mass of an atom is obtained by dividing the atomic mass by Avogadro’s number.
Question: Calculate the number of moles in 12.044 ××1023 helium atoms.
Answer: Given : N = 12.044 × 1023 (of Helium)
N0 = 6.022 × 1023, n = ?
We know that,
No. of moles (n) = No.of particles(N )/Avogadro’s number ( NO ) or n =N/No
∴ n=12. 044x 1023/6. 022×1023=2
Thus, number of moles in 12.044 × 1023 helium atoms is 2.
Question: What are the postulates of Dalton’s atomic theory. Give at least four points.
Answer: The postulates of Dalton’s atomic theory may be stated as follows :
(i) All matter is made of very tiny particles called atoms.
(ii) Atoms are indivisible particles, which cannot be created or destroyed in a chemical reaction.
(iii) Atoms of a given element are identical in mass and chemical properties.
(iv) Atoms of different elements have different masses and chemical properties.
(v) Atoms combine in the ratio of simple whole number to form compounds.
(vi) The relative number and kinds of atoms are constant in a given compound.
Question: How many ions are there in 80 g of magnesium oxide?
Answer: Mass of magnesium oxide/ Molar mass of magnesium oxide=80/40=2 MgO →Mg2+ + O2–
From the equation, we can see that 1 mol of MgO contains
1 mol of Mg2+ ions and 1 mol of O2– ions. 2.0 mol of MgO
will contain 2.0 mol of Mg2+ ions and 2.0 mol of O2– ions.
Hence, 2.0 mol of MgO contains 4.0 mol of ions.
Number of ions = 4 × 6.022 × 1023 = 2.4088 × 1024
Question: Carbon and oxygen react with each other in the ratio 3 : 8 by mass. What weight of carbon should be used to react completely with 40 g of oxygen?
Answer: Ratio by mass C : O
3 8
? 40
Mass of carbon required =3 x40/8=15g
Question: a. Define atomic mass unit.
b. What is meant by saying that “the atomic mass of oxygen is 16”?
Answer: (a) The atomic mass unit is defined as1/12th of the mass of one atom of a carbon (C–12) isotope.
(b) It means that one atom of oxygen is 16 times heavier than 1/12th of the mass of a 12C atom.
Question: How many moles are present in 11.5 g of sodium?
Answer: No. of moles in 11.5 g of sodium = Givenmass (in g)/Molar mass
= 11. 5/23=0. 5
Question: A piece of copper weighs 0.635 g. How many atoms of copper does it contain?
Answer: Gram atomic mass of copper = 63.5 g
Number of moles in 0.635 g of copper = 0 635/63 5=0. 01moles
Number of copper atoms in one mole = 6.022 × 1023
Number of copper atoms in 0.01 moles
= 0.01 × 6.022 × 1023 = 6.022 × 1021
Question: Convert into mole.
a. 12 g of oxygen gas
b. 20 g of water
c. 22 g of carbon dioxide
Answer: (a) Molar mass of O2 = 2 × 16 = 32 g
∴ Number of moles in 12 g of O2
= Given mass/Molar mass = 12/32=0.375
(b) Molar mass of H2O = 2 × 1 + 16 = 18 g
∴ Number of moles in 20 g of H2O = 20/18=1.11 moles
(c) Molar mass of CO2 = 12 + 16 × 2 = 12 + 32 = 44 g
∴ Number of moles in 22 g of CO2 =22/44= 0.5 mole
Question: An element X shows a variable valency of 3 and 5. What are the formulae of the oxide formed by it?
Answer:
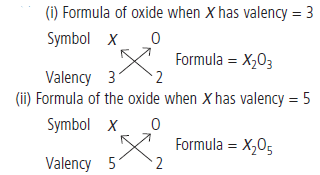
Question: What mass of silver nitrate will react with5.85 g of sodium chloride to produce 14.35 g ofsilver chloride and 8.5 g of sodium nitrate if the law of conservation of mass is true?
Answer: The reaction is Silver nitrate + Sodium chloride → Silver chloride + Sodium nitrate
If law of conservation of mass is true,Total mass of reactants = Total mass of products
i.e., Mass of AgNO3 + Mass of NaCl = Mass of AgCl +Mass of NaNO3
We have : Mass of NaCl = 5.85 g, Mass of AgCl = 14.35 g,Mass of NaNO3 = 8.5 g
Substituting these values in the above equation, we get Mass of AgNO3 + 5.85 g = 14.35 g + 8.5 g
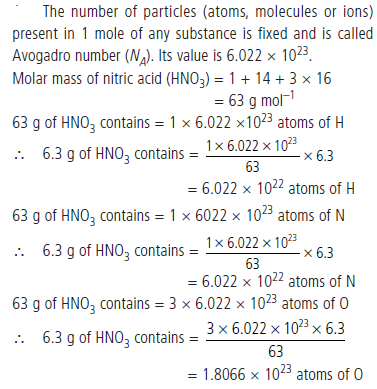
Question: What is Avogadro number? How many atoms of each element are present in 6.3 g of nitric acid (HNO3)?
Answer:
Question: Calculate the formula unit masses of ZnO,
Na2O, K2CO3. Given atomic masses of Zn = 65
u, Na = 23 u, K = 39 u, C = 12 u and O = 16 u.
Answer: Formula unit mass of ZnO = atomic mass of Zn
+ atomic mass of O = (65 + 16) u = 81 u
Formula unit mass of Na2O = 2 × atomic mass of Na
+ atomic mass of O = (2 × 23 + 16) u = 62 u
Formula unit mass of K2CO3 = 2 × atomic mass of
K + atomic mass of C + 3 × atomic mass of O
= (2 × 39 + 12 + 3 × 16) u = (78 + 12 + 48) u = 138 u
Question: 4.9 g of sulphuric acid contains 0.1 g of hydrogen, 1.6 g of sulphur and rest oxygen.
Calculate the mass percentage composition of all the elements of sulphuric acid.
Answer:

Question: Calculate the number of aluminium ions present in 0.051 g of aluminium oxide.
(Hint : The mass of an ion is the same as that of an atom of the same element. Atomic mass of Al = 27 u)
Answer: Molar mass of Al2O3 = 2 × 27 + 3 × 16 = 54 + 48 = 102 g
102 g of Al2O3 contains = 2 × 6.022 × 1023 Al3+ ions
∴ 0.051g of Al2O3 contains = 2x 6.022 x1023/102 x 0.051.
= 6.022 × 1020 Al3+ ions
Question: 1022 atoms of an element ‘X’ are found to have a mass of 930 mg. Calculate the molar mass of the element ‘X’.
Answer:

Question: A 0.24 g sample of compound of oxygen and boron was found by analysis to contain 0.096 g of boron and 0.144 g of oxygen. Calculate the percentage composition of the compound by weight.
Answer: We know that, % of any element in a compound
= Mass of element/Mass of compound x100
% of boron = 0. 096/0. 24 x100 =40%
% of oxygen = 0 .144/0 .24 x 100= 60%
Question: The molecular formula of ferric sulphate is Fe2(SO4)3. (Atomic mass : Fe = 56 u, S = 32 u,
O = 16 u)
a. Calculate the molar mass of Fe2(SO4)3.
b. How many moles of each element are there in 40 g of ferric sulphate?
Answer: (a) The molar mass of Fe2(SO4)3
= 2 × 56 + 3 × 32 + 12 × 16 = 400(b) No. of moles = Given mass/Molar mass= 40/400=0 1 .
1 mole of ferric sulphate contains 2 moles of iron
0.1 mole of ferric sulphate contains = 2 × 0.1
= 0.2 moles of Fe
1 mole of ferric sulphate contains 3 moles of sulphur
0.1 mole of ferric sulphate contains = 3 × 0.1
= 0.3 moles of S
1 mole of ferric sulphate contains 12 moles of oxygen
0.1 mole of ferric sulphate contains = 12 × 0.1
= 1.2 moles of O
Question: a. An element shows variable valencies 4 and 6. Write the formulae of its two oxides.
b. An element forms an oxide A2O5.
(i) What is the valency of the element A?
(ii) What will be the formula of the chloride of the element?
Answer: (a) Let the element be represented by the symbol E.
Formula of oxide in which valency of E = 4 are E2O4 or EO2
Formula of oxide in which valency of E = 6 are E2O6 or EO3
(b) Formula of oxide of the element = A2O5
(i) The valency of the element A in the oxide = 5
(ii) The formula of the chloride of the element A = ACl5
Question: What is the mass of
a. 1 mole of nitrogen atoms?
b. 4 moles of aluminium atoms (atomic mass of aluminium = 27)?
c. 10 moles of sodium sulphite (Na2SO3)? C
Answer: (a) Mass of 1 mole nitrogen atoms
= number of moles × atomic mass = 1 × 14 = 14 g
(b) Mass of 4 moles aluminium atoms = 4 × atomic mass
= 4 × 27 = 108 g
(c) Mass of Na2SO3 = 2 × 23 + 32 + 16 × 3
= 46 + 32 + 48 = 126 g
1 mole of Na2SO3 = 126 g
∴ 10 moles of Na2SO3 = (10 × 126) g = 1260 g
Question: Calculate the number of atoms of each element present in 122.5 g of KClO3.
Answer: No. of moles of KClO3= = 122. 5/122. 5=1
(mol. wt. of KClO3 = 122.5)
From the formula KClO3, we know that 1 mole of KClO3
contains 1 mole of K atoms, 1 mole of Cl atoms and 3 moles
of O atoms.
∴ No. of atoms of K = 6.022 × 1023
No. of atoms of Cl = 6.022 × 1023
No. of atoms of O = 3 × 6.022 × 1023 = 18.066 × 1023
Question: Which amongst the following has more number of atoms?
(i) 11.5 g of sodium or
(ii) 15 g of calcium [Na = 23; Ca = 40] A
Answer: We know that equal number of moles of different elements contain equal number of atoms. Thus, we shall convert masses of sodium and calcium to find which has more number of moles.
(i) For sodium
Gram atomic mass of sodium = 23 g
Mass of sodium = 11.5 g
∴ No. of moles of sodium = Mass of sodium ingrams/Gram atomic mass of sodium
= 11.5 g /23 g == 0.5 moles
(ii) For calcium
Gram atomic mass of calcium = 40 g
Mass of calcium = 15 g
∴ No. of moles of calcium
= Mass of calciumingrams /Gram atomicmass of calcium = 15 g/40 g= 0.375 moles
Therefore, sodium has more number of atoms than calcium.
Question: A flask P contains 0.5 mole of oxygen gas.
Another flask Q contains 0.4 mole of ozone gas.
Which of the two flasks contains greater number of oxygen atoms?
Answer: In flask P :
1 molecule of oxygen (O2) = 2 atoms of oxygen
1 mole of oxygen gas = 6.022 × 1023 molecules
0.5 mole of oxygen gas = 6.022 × 1023 × 0.5 molecules
= 6.022 × 1023 × 0.5 × 2 atoms = 6.022 × 1023 atoms
In flask Q :
1 molecule of ozone (O3) = 3 atoms of oxygen
1 mole of ozone gas = 6.022 × 1023 molecules
0.4 mole of ozone gas = 6.022 × 1023 × 0.4 molecules
= 6.022 × 1023 × 0.4 × 3 atoms = 7.23 × 1023 atoms
∴ Flask Q has a greater number of oxygen atoms as compared
to flask P.
Long Answer Type Question
Question : Calculate the number of moles of phosphorus (P) atoms in 100 g of phosphorus. If phosphorus is considered to contain P4 molecules, then how many moles of P4 molecules are there?
Answer: Mass of phosphorus = 100 g
Atomic mass of P = 31.0 u
So, molar mass of P atom = 31 g
Therefore,
Number of moles of P atoms in 100 g of phosphorus
= 100/31 0=3. 22 mol
If phosphorus is considered to be present as P4 molecules,
then
No. of moles of P4 molecules in 100 g of phosphorus
= No. of molesofP atoms in100 gof phosphorus/4
= 3. 22 mol/4 = 0.805 mol
Thus, 100 g of phosphorus contains 3.22 moles
of P atoms and 0.805 moles of P4 molecules.
Question : 25.4 g of iodine and 14.2 g of chlorine are made to react completely to yield a mixture of ICl and ICl3. Calculate the ratio of the moles of ICl and ICl3.
Answer:
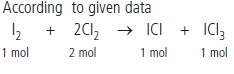
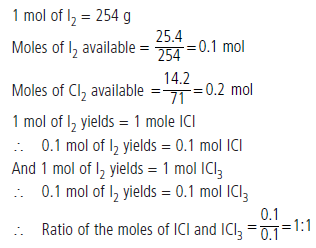
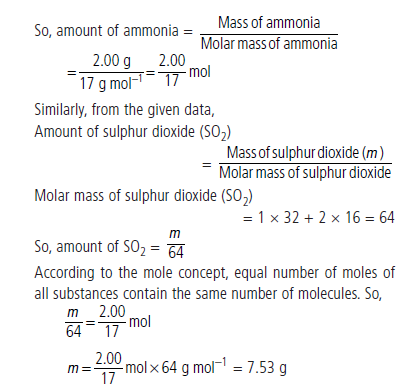
Question : A sample of ammonia (NH3) weighs 2.00 g.
What mass of sulphur dioxide (SO2) contains the same number of molecules as are in 2.00 g of ammonia?
Answer:
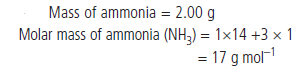
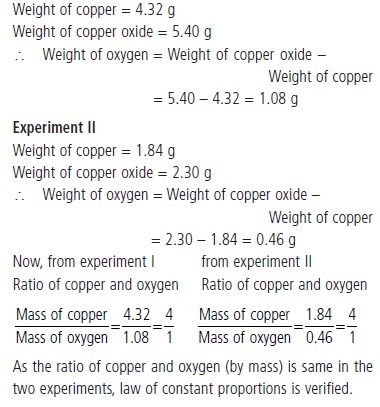
Question : Weight of copper oxide obtained by heating 4.32 g of metallic copper with nitric acid was 5.40 g. In another experiment, 2.30 g of copper oxide on reduction yielded 1.84 g of copper. Show that these findings are in accordance with the law of constant proportions.
Answer:
Question : (i) What is the mass of one atom of hydrogen? (atomic mass of hydrogen = 1 u)
(ii) How many NH4+ ions are present in 1.5 moles of (NH4)3PO4?
Answer: (i) Mass of 6.022 × 1023 hydrogen atoms = 1 g
Mass of 1 hydrogen atom =1g /6 .022x 1023
= 1.66 × 10–24 g = 1.66 × 10 –27 kg
(ii) Number of (NH4)3PO4 molecules= 1.5 × 6.022 × 1023
One molecule of (NH4)3PO4 contains 3 NH4++ ions. Hence, total
number of NH4 + ions
= 3 × 1.5 × 6.022 × 1023 = 27.1 × 1023

