Class 12 Chemistry Sample Paper Set D
Please see below Class 12 Chemistry Sample Paper Set D with solutions. We have provided Class 12 Chemistry Sample Papers with solutions designed by Chemistry teachers for Class 12 based on the latest examination pattern issued by CBSE. We have provided the following sample paper for Class 12 Chemistry with answers. You will be able to understand the type of questions which can come in the upcoming exams.
CBSE Sample Paper for Class 12 Chemistry Set D
1. In cyclotrimetaphosphoric acid, number of P—O—P bonds, P = O bonds and P—OH bonds are respectively
(a) 6, 3, 3
(b) 5, 0, 3
(c) 4, 3, 0
(d) 3, 3, 3
Answer
D
2. Which of the following carbonyl compounds is most polar?

Answer
D
3. In an irreversible process taking place at constant T and P and in which only pressure volume work is being done, the change in Gibbs free energy (dG) and change in entropy (dS), satisfy the criteria
(a) (dS)V, E < 0, (dG)T, P < 0
(b) (dS)V, E > 0, (dG)T, P < 0
(c) (dS)V, E = 0, (dG)T, P = 0
(d) (dS)V, E = 0, (dG)T, P > 0
Answer
B
4. The reagent with which both acetaldehyde and acetone react easily is
(a) Fehling’s reagent
(b) Grignard reagent
(c) Schiff’s reagent
(d) Tollens’ reagent.
Answer
B
5. If the solubility of PbCl2 at 25°C is 6.3× 10–3 mol L–1 its solubility product at that temperature is
(a) (6.3 × 10–3) × (6.3 × 10–3)2
(b) (6.3 × 10–3) × (12.6 × 10–3)2
(c) (6.3 × 10–3) × (12.6 × 10–3)
(d) (12.6 × 10–3) × (12.6 × 10–3)
Answer
B
6. Which of the following organic compounds gives positive Fehling’s test as well as iodoform test?
(a) Methanal
(b) Ethanol
(c) Propanone
(d) Ethanal
Answer
D
7. What is the maximum number of emission lines when the excited electron of a hydrogen atom in n = 6 drops to ground state?
(a) 6
(b) 15
(c) 30
(d) 10
Answer
B
8. Which one of the following species is not a pseudohalide?
(a) CNO–
(b) RCOO–
(c) OCN–
(d) NNN–
Answer
B
9. The number of radial nodes of 3s and 2p orbitals are respectively
(a) 2, 0
(b) 0, 2
(c) 1, 2
(d) 2, 1
Answer
A
10. The number of structural and configurational isomers of a bromo compound C5H9Br, formed by the addition of HBr to pent-2-yne respectively are
(a) 1 and 2
(b) 2 and 4
(c) 4 and 2
(d) 2 and 1
Answer
B
11. Alkali halides do not show Frenkel defect because
(a) cations and anions have almost equal size
(b) there is a large difference in size of cations and anions
(c) cations and anions have low coordination number
(d) anions cannot be accommodated in voids.
Answer
A
12. The structure of 2R, 3S-dibromocinnamic acid is

Answer
D
13. The EMF of the cell, Zn | Zn2+ (0.01 M) | | Fe2+ (0.001M) | Fe at 298 K is 0.2905 V then the value of equilibrium constant for the cell reaction is
(a) e0.32/0.0295
(b) 100.32/0.0295
(c) 100.26/0.0295
(d) 100.32/0.0591
Answer
B
14. Interhalogen compounds are more reactive than the individual halogens because
(a) they are prepared by direct combination of halogens
(b) X – X′ bond is weaker than X – X or X′– X′ bond
(c) they are thermally more stable than halogens
(d) there is a large difference in their electronegativities.
Answer
B
15. Ground state energy of H-atom is (– E1), the velocity of photoelectrons emitted when photon of energy E2 strikes stationary Li2+ ion in ground state will be

Answer
C
16. Oils are converted into fats by
(a) hydration
(b) decarboxylation
(c) hydrogenation
(d) dehydrogenation.
Answer
C
17. Glycol is added to aviation petrol because
(a) it prevents freezing of petrol
(b) it minimises the loss of petrol
(c) it increases the efficiency of fuel
(d) it prevents the engine from heating up.
Answer
A
18. Which of the following expressions correctly represents the equivalent conductance at
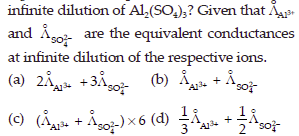
Answer
B
19. The correct order of ionic radii of Ce, La, Pm and Yb in +3 oxidation state is
(a) La3+ < Pm3+ < Ce3+ < Yb3+
(b) Yb3+ < Pm3+ < Ce3+ < La3+
(c) La3+ < Ce3+ < Pm3+ < Yb3+
(d) Yb3+ < Ce3+ < Pm3+ < La3+
Answer
B
20. The IUPAC name of the following compound
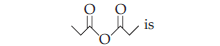
(a) propionic anhydride
(b) dipropanoic anhydride
(c) ethoxy propanoic acid
(d) propanoic anhydride.
Answer
D
21. Consider the reactions,

Answer
A
22. The major product of the following reaction is

Answer
B
23. Which of the following arrangements shows schematic alignment of magnetic moments of antiferromagnetic substances?

Answer
D
24. The ease of liquefaction of noble gases decreases in the order
(a) He > Ne > Ar > Kr > Xe
(b) Xe > Kr > Ar > Ne > He
(c) Kr > Xe > He > Ar > Ne
(d) Ar > Kr > Xe > He > Ne
Answer
B
25. Consider the following sets of quantum numbers:

Which of the following sets of quantum numbers is not possible?
(a) (i), (ii), (iii) and (iv)
(b) (ii), (iv) and (v)
(c) (i) and (iii)
(d) (ii), (iii) and (iv)
Answer
B
26. Which of the following is not formed when glycerol reacts with HI?
(a) CH2 = CH – CH2I
(b) CH2OH – CHI – CH2OH
(c) CH3 – CH = CH2
(d) CH3 – CHI – CH3
Answer
B
27. For preparing a buffer solution of pH = 6 by mixing sodium acetate and acetic acid, the ratio of the concentration of salt and acid should be (Ka = 10–5)
(a) 1 : 10
(b) 10 : 1
(c) 100 : 1
(d) 1 : 100
Answer
B
28. Which one of the following is hydride transfer reaction?

Answer
A
29. Which of the following compounds can be detected by Molisch’s test?
(a) Sugars
(b) Amines
(c) Primary alcohols
(d) Nitro compounds
Answer
A
30. Hess’s law is applicable for the determination of heat of
(a) transition
(b) formation
(c) reaction
(d) all of these.
Answer
D
31. Which of the following statements is true regarding main cause of lanthanide contraction?
(a) Poor shielding of 4f-electron by another in the subshell
(b) Poor shielding of 5d-electrons
(c) Effective shielding of 4f-electrons
(d) Effective shielding of 5d-electrons by 4f-electrons
Answer
A
32. Which of the following will be most readily dehydrated in acidic conditions?

Answer
A
33. An organic compound with the formula C6H12O6 forms a yellow crystalline solid with phenylhydrazine and gives a mixture of sorbitol and mannitol when reduced with sodium. Which among the following could be the compound?
(a) Fructose
(b) Glucose
(c) Mannose
(d) Sucrose
Answer
A
34. Which of the following statements is incorrect?
(a) t1/2 ∝ a, for zero order reaction.
(b) t1/2 is independent of a, for first order reaction.
(c) t1/2 ∝ 1/a2 , for third order reaction.
(d) t1/2 ∝ 1/a1−n , for nth order reaction
Answer
D
35. An ester (A) with molecular formula C9H10O2 was treated with excess of CH3MgBr and the compound so formed was treated with conc. H2SO4 to form olefin (B). Ozonolysis of (B) gave ketone with formula C8H8O which shows positive iodoform test. The structure of (A) is
(a) C6H5COOC2H5
(b) CH3OCH2COC6H5
(c) CH3COC6H4COCH3
(d) C6H5COOC6H5
Answer
A
36. In the reduction of nitrobenzene, which of the following is the intermediate?
(a) C6H5 – N = O
(b) C6H5NH – NHC6H5
(c) C6H5 – N = N – C6H5
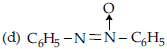
Answer
A
37. Which of the following ligands will not show chelation?
(a) EDTA
(b) DMG
(c) Ethene-1, 2-diamine
(d) SCN–
Answer
D
38. pKa of a weak acid is 5.76 and pKb of a weak base is 5.25. What will be the pH of the salt formed by the two?
(a) 7.255
(b) 7.005
(c) 10.225
(d) 4.255
Answer
A
39. Which of the following compounds does not contain chiral carbon?
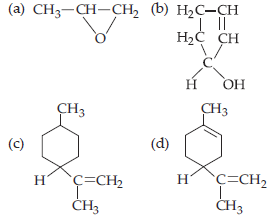
Answer
C
40. Increasing order of reactivity of the following alkyl halides in the Williamson’s synthesis is
I. CH2 = CHCH2Cl
II. CH3CH2CH2Br
III. (CH3)3CCH2Br
IV. CH3CH2CH2Cl
(a) II < III < IV < I
(b) III < II < IV < I
(c) IV < III < I < II
(d) III < IV < I < II
Answer
D
