Acids Bases Salts Notes for Class 10 Science

1. Acids: Those substances which turn blue litmus solution red are called acids. The term ‘acid’ has been derived from the Latin word ‘acidus’ which means sour. Acids are sour in taste. They give ions in aqueous solution, e.g., HCL (Hydrochloric acid). (Sulphuric acid), (Nitric acid), (Acetic acid), (Carbonic acid), (Sulphurous acid) , HCN (Hydrocyanic acid), (Boric acid), (Phosphoric acid), ( ) (Oxalic acid), HBr (Hydrobromic acid), HI (Hydroiodic acid), HF (Hydrofluoric acid). Note: Concentrated Acid-Those acid which contains minimum amount of water eg Sulphuric acid (concentration 98 %) HCl(39 %) are known as concentrated acid.
1. Indicators: Those substances which change their colour in different types of substances are called indicators. There are many natural substances like red onion peels, red cabbage leaves, beetroot extract, coloured petals of some flowers like rose, Petunia, Hydrangea and Geranium, Bougainvillea, turmeric which indicate the presence of sour substances (acids) and bitter substances (bases). They are called indicators because they indicate the presence of acid or base by showing change in colour.
Note: Synthetic Indicator –Methyl orange, Phenolphthalein 2. Litmus Solution: It is purple dye which is extracted from plant ‘lichen’. It is used as acid-base indicator. It is available in the lab as blue or red litmus solution. When purple solution is acidified, it changes to blue colour (called blue litmus solution) whereas it changes to red colour when a small amount of base is added to blue litmus solution (called red litmus solution). Note: .Litmus solution is a purple dye extracted from lichen.
3. Bases:

Those substances which change red litmus solution blue are called bases. They are bitter in taste. They are present in bitter substances like bitter gourd, baking soda solution, neem cucumber extract, washing soda solution, slaked lime solution and soap solution. They give OH– ions in aqueous solution, e.g., NaOH, KOH, . Soluble bases are called alkalies, e.g., NaOH, KOH,NH4 OH ,Ca (OH )2 .
Note: Some bases are called alkalies.
4. Acid-Base Indicators: Those substances which change their colour in acid and base are called acidbase indicators, e.g., Litmus solution is used as acid-base indicator. Blue litmus hanges to red in acidic solution whereas red litmus solution becomes blue in basic solution. Turmeric also acts as indicator. You must have observed if your clothes have yellow spot due to turmeric it becomes red when washed with soap solution showing that turmeric changes its colour in basic solution, therefore, can act as indicator.
5. Precautions to be used while handling Acids in the Laboratory: All living things are very sensitive to acids and too much acid can kill cells or stop proper working of the cells. oncentrated acids are very dangerous and should never be handled without protection. The following precautions must be observed while dealing with acids.
I. Never try to touch or taste acids which are used in laboratory.
II. Never add water into conc. acid otherwise bottle will break. Always add conc. acid to water very slowly with continuous cooling under running water.
6. Advantages of Acids:
I. Hydrochloric acid is released in stomach to make medium acidic in nature. It leads to coagulation of proteins and helps in their digestion.
II. HCl kills bacteria coming to the stomach along with the food.
III. Vinegar (Acetic acid) is used as preservative in pickles and in Chinese food. It gives sour taste to food.
IV. Cold drinks contain carbonic acid.
V. Lemon contains citric acid which is used in case of indigestion.
VI. Oranges and amla contain ascorbic acid (Vitamin C) which prevents scurvy.

7. Concentrated Acid: Those acids which contain minimum amount of water are called concentrated acids. Conc. HCl is 39% by ass, conc. HNO3 is 69% by mass and conc. H2SO4 is 98% by mass. Note: Pure acetic acid (100%) is called glacial acetic acid.
8. Synthetic Indicators: Those chemical substances which change their colour in acids and bases are called synthetic indicators. They are also called synthetic acid-base indicators, e.g., methyl orange, phenolphthalein, methylene blue, gentian violet, methyl red, etc.
Note: Synthetic Indicator –Methyl orange, Phenolphthalein
9. Natural indicator :Red cabbage leaves ,red onion peels ,turmeric and coloured petals of some flowers like Hydrangea ,petunia, beet root all are natural indicators.
10. Reaction of Metals with dil. HCl:
Reactive metals react with dil. HCl to liberate hydrogen gas.
Zn(s) + 2HCl (aq) → ZnCl2 (aq) + H2 (g)
2Al(s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2 (g)
Mg(s) + 2HCl (aq) → MgCl2 (aq) + H2 (g)
2Na(s) + 2HCl (dil) → 2NaCl (aq) + H2 (g)
Ca(s) + 2HCl (dil) → CaCl2 (aq) + H2 (g)
2K(s) + 2HCl (dil) → 2KCl (aq) + H2 (g)
The order of reactivity is
K > Ca > Na > g > Al > Zn > Fe > Sn > Ph > H > Cu > Ag > Au > Pt.
Copper, silver, gold, mercury, platinum do not liberate hydrogen gas from dil. H2SO4 or dil. HCl because they are less reactive than hydrogen.

Note: Acidic salts pH>7: Basic salts pH>7:Neutral salts=7 Neutral salts are salts of strong acid and strong bases.
11. Some Common Characteristics of Acids:
• All acids contain hydrogen.
• They react with reactive metals to liberate hydrogen gas.
• But all compounds containing hydrogen cannot be acid, e.g., CH4, C2H6, C3H8, C6H12O6, C12H22O11 contain hydrogen but are not called acids.
12. Hydronium Ions: They are formed by reaction of (from acid) and H2O. It is because is unstable. It reacts with H2O to from (Hydronium ion).
H++ H2O →H3O+
13. Ionisation: The process of forming ions in aqueous solution is called ionisation. All ionic compounds like HCl, HNO3, H2SO4, CH3COOH form ions in aqueous solution.
14. Universal Indicator: A universal indicator is a mixture of indicators which shows a gradual but well marked series of colour changes over a very wide range of change in concentration of ions.
15. pH Scale: It is scale for measuring hydrogen ion concentration. The concentrations of are generally small, therefore concentrations of are expressed in terms of pH. pH is defined as negative logarithm of concentration or concentration.
pH=-log[H+] or pH=-log[H3O+]
pH stands for power of hydrogen.
pH < 7 is acidic, pH = 7 is neutral, pH > is basic.
16. pH Paper: The paper which is coated with universal indicator is called pH paper.
17. Relation between conc. and pH: pH=-log[H3O]
Higher the concentration of , lower will be pH. pH paper or universal indicator solution is used to determine approximate pH of solution. Litmus solution can only tell us that the solution is acidic or basic.
18. pH of Bases: Basic solution also have H+ ions but still they are bases because [OH–]is more than [H+] , therefore, pH of bases is more than 7.
19. Applications of pH in Daily Life:
I. Plants grow in soil having specific pH.
II. pH of stomach is 2.0 due to secretion of HCl. In case of indigestion, acidity increases which can be neutralized by antacids like milk of magnesia, baking soda, which neutralize excess of acid and give relief rom pain. Antacids like milk of magnesium and sodium bicarbonate are weak bases.
III. Digestion of carbohydrates does not take place in stomach due to acidic pH in which salivary amylase becomes inactive. It takes place in small intestine where pH is basic.
IV. Cold drinks, chocolates and sweets are most harmful for health as well as tooth. They produce acids in mouth which are responsible for tooth decay. You may be surprised to know that tooth gets dissolved in cold drink if kept for long time. Therefore, we must avoid cold drinks and sweets and should brush our teeth after every meal so as to prevent tooth decay.
20. Nature of Soil for Crops: Usually neutral soil is best for crops. If soil is acidic, farmers treat the soil with quicklime (CaO) or slaked lime Ca(OH)2 or CaCO3 (Chalk) so as to make it neutral. Acidic soil is mostly harmful for crops. Alkaline soil is also not suitable for plants.
21. Strong Acids: Those acids which dissociate into ions completely are called strong acids, e.g., H2SO4, HCl, HBr, Hl, HNO3, HClO4 (Perchloric acid) are strong acids.
(a) HCl → H+CL– (b) HBr → H++Br- (c) Hl → H++1– (d) HNO3 → H++NO–3 (e) H2SO4 → 2H+SO2-4 (f) HClO4 → H++ClO
Single arrow head means complete ionisation.
22. Weak Acids: Those acids which do not dissociate into ions completely are called weak acids, e.g., citric acid, acetic acid, ascorbic acid, tartaric acid, formic acid.

The double arrow head shows the ionisation is not complete and acid is weak.
23. Neutralization Reaction: The reaction in which base or basic oxide reacts with acid or acidic oxide is called neutralization reaction. e.g.,

24. Examples of Neutralization Reactions:

25. Displacement Reactions: Those reactions in which more reactive metal can displace less reactive metal from its salt solution.
26. Chemical in Common Salt: The main chemical present in common salt is sodium chloride. It is obtained by neutralization reaction of sodium hydroxide with HCl (Hydrochloric acid). It is obtained on a large scale from sea water. It is found in large deposits called rock salt.
27. Uses of Common Salt:
I. It is used in daily food.
II. It is used as preservative.
III. It is used for manufacture of Na metal and Cl2 (g) by electrolysis in molten state.
28. Manufacture of Sodium Hydroxide: Sodium hydroxide is the most important alkali and is made commercially by electrolysis of saturated brine solution (sodium chloride). This process is called chlor-alkali process
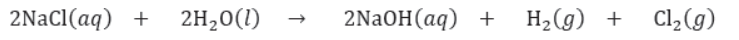
29. Washing Soda: Its chemical formula is Na2CO3, 10H2O, e.g., sodium carbonate dehydrate i.e., one mole of Na2CO3 contains 10 moles of water of crystallization.
Anhydrous sodium carbonate is called soda ash.
Sodium carbonate is crystallized by dissolving in water to get washing soda.
30. Displacement Reactions: Those reactions in which more reactive metal can displace less reactive metal from its salt solution.

31. Common Salt (NaCl)
1.formed by reaction of Sodium hydroxide(NaOH) and
2.Rock salt
3.Neutral salt
32. Uses of Common Salt:
• It is used in daily food.
• It is used as preservative
• It is used for manufacture of Na metal and Cl2 (g) by electrolysis in molten state.
33. Manufacture of Sodium Hydroxide: Sodium hydroxide is the most important alkali and is made commercially by electrolysis of saturated brine solution (sodium chloride). This process is called chlor-alkali process
34.Washing Soda: Its chemical formula is Na2CO3. 10H2O, e.g., sodium carbonate decahydrate i.e., one mole of Na2CO3 contains 10 moles of water of crystallization.
Anhydrous sodium carbonate is called soda ash.
Sodium carbonate is crystallized by dissolving in water to get washing soda.

35. Properties of Washing Soda:
I. It is a transparent crystalline solid.
II. It contains ten molecules of water of crystallization.
III. Washing soda dissolves in water to from an alkaline solution which turns red litmus blue. It shows that its aqueous solution is alkaline in nature.
IV. When treated with HCl or H2SO4 it liberates CO2 gas.
V. When CO2 gas is passed through aqueous solution of sodium carbonate, sodium hydrogen carbonate gets precipitated.
36. Uses of Sodium Carbonate:
I. It is used in manufacture of glass, soap, paper and other sodium compounds like borax, caustic soda, etc.
II. It is used in softening of hard water.III. It is used as washing soda in laundries.
IV. It is used as cleaning agent for domestic purposes.
37. Baking Soda (NaHCO3): Baking soda chemically is sodium hydrogen carbonate.
It is prepared by passing CO2(g) through aqueous solution of Na2CO3

I. Its aqueous solution is alkali8ne in nature due to hydrolysis. The solution is weakly basic.

This solution gives yellow colour with methyl orange (indicator) but no colour with phenolphthalein.
II. On heating, it loses carbon dioxide and water forming sodium carbonate.

III. When it comes in contact with H2SO4 it gives CO2 which is used in fire extinguishers.

38. Uses of Sodium Hydrogen carbonate:
I. It is used as antacid (medicine) under the name soda bicarbonate to neutralize excess of acidity (hyperacidity) in the stomach.
II. It is an ingredient of baking powder which contains NaHCO3 and tartaric acid. When baking powder is heated, sodium hydrogencarbonate decomposes to give CO2 and sodium carbonate.
CO2 causes bread and cake to rise. Tartaric acid helps to remove bitter taste due to formation of Na2CO3.
39. Bleaching Powder (CaOCl2): Chemically, it is called calcium oxychloride. It is also called as chloride of lime.
Manufacture: It is manufactured by Hasenclever’s plant or in Bachmann’s plant by the reaction of dry slaked lime with chlorine gas.

40. Properties of Bleaching Powder (Calcium oxychloride):
I. It is a pale yellow powder. It has a strong sell of chlorine.
II. It is soluble in water but a clear solution is never formed due to presence of impurities.
III. It loses chlorine by the action of carbon dioxide.

41. Uses of Bleaching Powder:
I. It is used for bleaching cotton. Linen in textile industries, for bleaching washed clothes in laundry.
II. It is used as oxidising agent in many chemical industries.
III. It is used for disinfecting drinking water to make water free fro micro-organisms.
IV. It is used for manufacture of chloroform.
V. It makes wool unshrinkable.
42. Plaster of Paris(CaSO4.1/2H2O): Chemically Plaster of Paris is calcium sulphate hemihydrate. It is called Plaster of Paris because it is obtained from gypsum which is mainly found in Paris.
Preparation: Plaster of Paris is obtained by heating gypsum (CaSO4, 2H2O) at 373K in a kiln.
Heating should be done carefully.

43. Properties of Plaster of Paris:
I. It is white power.
II. When it is mixed with water, crystals of gypsum are produced and set into hard mass.

The setting process is exothermic, i.e., heat is evolved. The setting process may be catalyzed by sodium chloride while it can be retarded by borax or alum.
III. When Plaster of Paris is heated at 473K. it forms anhydrous calcium sulphate which is known as dead burnt plaster . It has no setting property as it takes up water very slowly.

44. Uses of Plaster of Paris:
I. It is used for plastering fractured bones and dislocated bones so as to set them in proper place.
II. It is used in making toys, decorative materials.
III. It is used in making casts for statues, toys, surgical instruments, etc.
IV. It is used in making blackboard chalks.
45. Uses of Mild Bases:
I. Washing soda is used as cleaning agent.
II. NaHCO3 acts a antacid.
III. Sodium carbonate is used in removing permanent as well as temporary hardness of water.
46. Water of Crystallization: It is fixed number of water molecules present in crystalline salt, e.g.,




