Biomolecules Notes Class 11 Biology
Please refer to the below Biomolecules Notes Class 11 Biology Chapter 8 prepared as per the latest syllabus and books issued for the current academic year. These revision notes have been prepared to help you understand all the difficult topics in this chapter. You will be able to easily understand and learn all important points so that you are able to get more marks in exams. We have provided Class 11 Biology Notes for all chapters on our website.
Class 11 Biology Chapter 9 Biomolecules Notes
Biomolecules
Biomolecule is an organic molecule that is produced by living organism. They act as building block of life and perform important functions in living organisms. They are primarily composed of carbon, hydrogen, nitrogen, oxygen, phosphorous and Sulphur. The four common biomolecules are- proteins, nucleic acid, carbohydrates, and lipids.
Analysis of chemical composition
For analysis of chemical composition of organic compound, living tissues are treated with trichloroacetic acid and grind it to form slurry. For inorganic chemical composition, sample of tissue should be burnt to obtain ash.
Proteins
Any of a class of nitrogenous organic compounds which have large molecules composed of one or more long chains of amino acids and are an essential part of all living organisms.
The building blocks of proteins are known as amino acids. There are 22 naturally occurring amino acids found in nature. Amino acids are made up of carbon, hydrogen, oxygen, and nitrogen. So, single amino acid is made up of amino group, carboxyl group, hydrogen atom and distinctive side chain, all bonded to alpha-carbon.
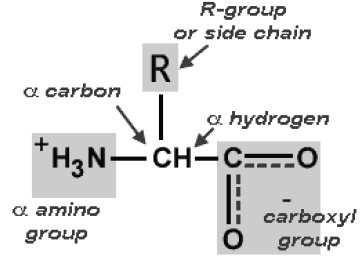
When amino acids are dissolved in water, it exists in solution as the dipolar ion or zwitterion. They can either act as proton donor or proton acceptor

All amino acids are optically active, that is, they can rotate the plane of polarized light. Optically active molecules contain chiral carbon except glycine. A tetrahedral carbon atom with four different constituents is said to be chiral.
Peptide is a compound consisting of two or more amino acids. When two amino acids are linked together via peptide bond, they are said to form a dipeptide. Three amino acids join to form tripeptide etc. Peptide chains of 12 to 20 amino acids form oligopeptide. When many amino acids are joined, they form polypeptide. The first amino acid is known as N terminal or amino terminal and last amino acid is said to be C terminal or carboxyl terminal.

Protein structure
Proteins have four levels of protein organization. They are as follows-
Primary structure of the protein is its sequence of amino acids. Amino acids are joined by a peptide bond.
Secondary structures are higher level of protein organization which includes- alpha helix and beta sheets. Both of them are stabilized by hydrogen bonds between carbonyl and N-H groups in a polypeptide backbone.
Alpha helix It is a rigid, rod like structure that forms when a polypeptide chain twists into a helical conformation.
Beta pleated sheets are formed when two or more polypeptide chain segment line up side by side. Each individual segment is known as beta strand.
Tertiary structures are the three-dimensional conformations that a protein assumes as a result of the interactions among the side chains in their primary structure. They are stabilized by hydrophobic interactions, electrostatic interactions, hydrogen bonds, van der waals forces and covalent bonds.
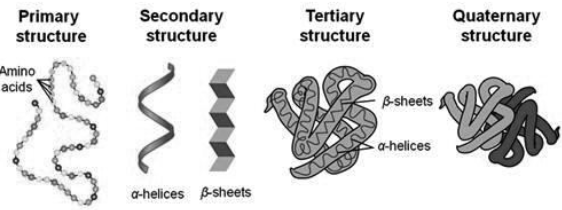
Quaternary structures : Quaternary structures are composed of two or more polypeptide chains. Polypeptides are held together via hydrophobic interactions, electrostatic interactions, and hydrogen bonds etc. For example, hemoglobin.
Fibrous and globular proteins:
Fibrous are long, rod shaped molecules which are insoluble in water. They are generally protective and structural in nature. Globular proteins are compact spherical molecules that are usually water soluble.
Nucleic acids
Nucleic acid was first discovered by Friedrich Miescher from the nuclei of pus cells. There two types of nucleic acids- deoxyribonucleic acids (DNA) and ribonucleic acids (RNA).
The monomeric unit of nucleic acid is known as nucleotides. Nucleotide is made up of three components- a nitrogenous base, a five-carbon sugar and phosphoric acid.
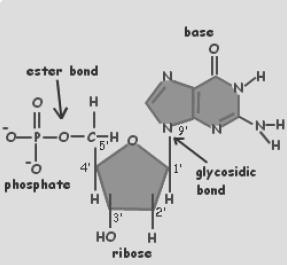
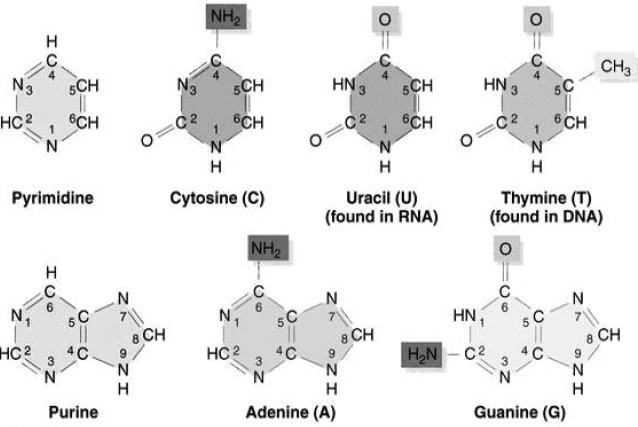
Nitrogenous bases are heterocyclic, aromatic molecules. There are basically two types of nucleic acids- purines and pyrimidines.
Purines are of two types- adenine and guanine whereas pyrimidines can be thymine, cytosine, or uracil.
The 5- carbon sugar found in DNA is deoxyribose and for RNA is ribose. Nucleotide without phosphate is known as nucleoside.
Structure of double stranded DNA
DNA are of different types such as-
B DNA
• Two long polynucleotide strands are coiled around the axis.
• Strands are antiparallel to each other.
• Guanine base pairs with cytosine and adenine base pairs with thymine.
• Guanine forms 3 hydrogen bonds with cytosine whereas adenine forms two hydrogen bonds with thymine.
Z DNA
• They are thinner than B DNA.
• They have alternating purine and pyrimidine bases.
• They are stabilized by high salt concentration.
Denaturation of DNA
When DNA is subjected to varying pH, temperature etc, two DNA strands separate out. That is the DNA is said to be denatured. If the denaturant such as temperature, pH is removed, the DNA regains its original or native structure. This is known as renaturation or annealing.
RNA
RNA known as ribonucleic acid. It usually exists as single strand. RNA exists in cells in various forms as explained below:
Messenger RNA carries genetic information from DNA in the form of codons (three nucleotides forms a codon), which codes for amino acids or protein.
Transfer RNA plays an important role during protein synthesis. They act as an interface between nucleic acid language and protein language. They are fund in the cytosol of the living cell.
Ribosomal RNA is a component of ribosomes. They have important roles during protein synthesis in eukaryotes and prokaryotes.
Carbohydrates
They are defined as the polyhydroxy aldehydes or polyhydroxy ketones. They consist of hydrogen, carbon, and oxygen. The simplest carbohydrates are known as monosaccharides, such as glucose. Two monosaccharides joined to form disaccharides. Polymers of 2 to 10 units of monosaccharides is known as oligosaccharides. When hundreds to thousands of monosaccharides are joined, they are known as polysaccharides. Monosaccharides with aldehyde group is known as aldoses. And monosaccharides with ketone group is known as ketoses. Trioses are the simplest monosaccharides.
Some common saccharides
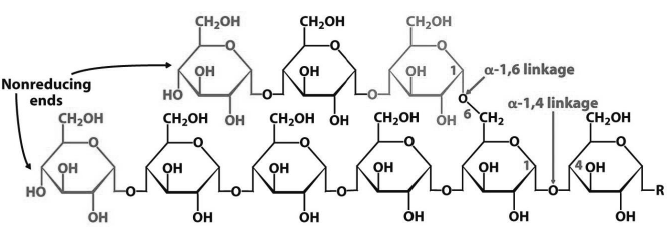
Starch : Starch is the storage form of energy is plants. It is a branched polysaccharide made up of glucose units. It is mixture of two different polymers amylose and amylopectin. Amylose is the unbranched polymer of glucose units whereas amylopectin is the branched polymer of glucose units.

Glycogen: Glycogen is the storage form of energy in animals. It is stored in muscle and liver. It is also a highly-branched polymer made up of amylose and amylopectin.
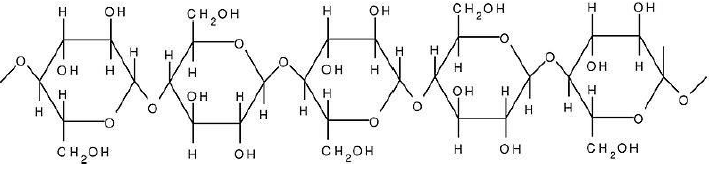
Cellulose : Cellulose is an unbranched polymer of glucose units. It is a structural polysaccharide of plant cells. It is the most abundant organic molecule in the biosphere. It is found in plant cells and provide strength and rigidity to the cell.
Chitin : Chitin is a linear polysaccharide composed of N-acetyl-D-glucosamine residues. It is found in the exoskeleton of insects and crustaceans.
Reducing and non-reducing sugar
Sugars capable of reducing ferric or cupric ion are called reducing sugar. All monosaccharides are reducing sugars.
Non-reducing sugars are not capable of reducing ferric or cupric ion. For example, sucrose is a non-reducing sugar.
Lipids
They are insoluble or poorly insoluble in water. They are soluble in non-polar solvents such as ether, chloroform or benzene.
Biological functions of lipids
• They serve as storage form of energy.
• They are major component of membranes.
• They are protective in function such as in bacteria, plants, insects, and vertebrates.
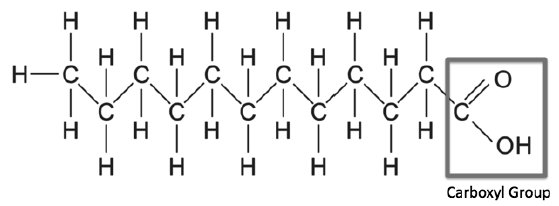
Fatty acids
They are the simplest form of lipids. They are composed of long chains of hydrocarbons with one carboxyl group. The alkyl chain may be saturated or unsaturated. Unsaturated fatty acids may contain one or more double bonds. They have both polar and non-polar ends. Mammals are unable to synthesize some fatty acids such as linoleate. Such fatty acids must be obtained from the diet, they are known as essential fatty acids. Some fatty acids can be synthesized endogenously, they are known as non-essential fatty acids.
Triacylglycerol also known as triglycerides are esters of fatty acids and glycerol. They are non-polar, hydrophobic in nature.
Enzymes
Almost all enzymes are proteins. Similar to protein, enzymes as primary, secondary and tertiary structure. Enzyme has an active site to which the substrate molecule can come and bind. The following are the properties of the enzymes-
• All enzymes are proteins, but all proteins are not enzymes.
• Enzymes are specific for their substrate.
• Enzymes acts as catalysts.
• They are not used up during the reaction.
• They are of six major types: oxidoreductases, transferases, hydrolases, lyases, ligases and isomerases.
• Some enzymes require a co-factor and/or a co-enzyme to function.
• Co-factor: Non-protein constituents that are bound to the enzyme and make the enzyme catalytically active.
• Co-enzyme: Organic compounds that bind to the enzyme transiently during the course of the reaction.
• Prosthetic groups: Organic substances that are bound very tightly to the enzyme
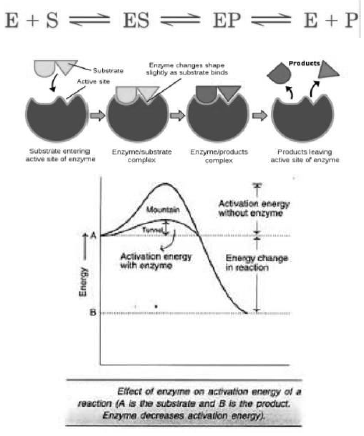
Factors affecting the enzyme activity
Temperature, pH and substrate concentration affects the enzyme activity. High or low temperature inactivates the enzyme activity. Deprotonation or protonation alters the enzyme activity.
Enzyme can also be inactivated by molecules known as inhibitors. The most common type of inhibitors are competitive inhibitors. These inhibitors compete with substrate for binding to the enzyme active site. For example, inhibition of succinate dehydrogenase by Malonate.

