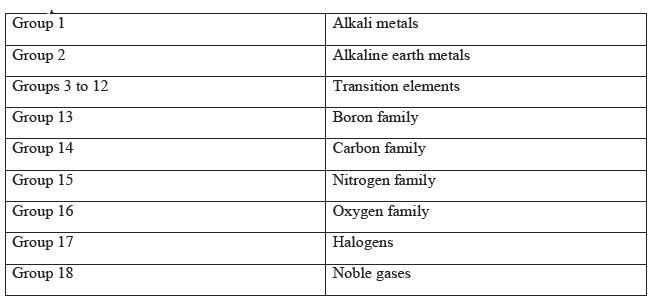
Periodic Classification of Elements Notes for Class 10 Science
Following are Periodic Classification of Elements Notes for Class 10 Science. These revision notes have been prepared by expert teachers of Class 10 Science as per the latest NCERT, CBSE, KVS books released for the current academic year. Students should go through Chapter 5 Periodic Classification of Elements concepts and notes as these will help you to revise all important topics and help you to score more marks. We have provided Class 10 Science notes for all chapters in your book. You can access it all free and download Pdf.
Chapter 5 Periodic Classification of Elements Notes Class 10 Science
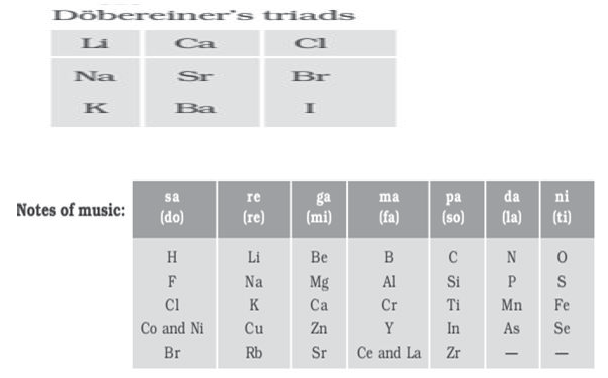
Dobereiner’s Triads
Dobereiner observed that when elements were arranged into groups of three in the order of their increasing atomic masses, the atomic mass of the middle element was the arithmetic mean of rest of the two.
Limitation
Could be applied only to limited number of elements. Only three sets could be identified.
Newlands’ Law of Octaves
Newlands found that every eighth element has chemical properties when they are arranged in increasing order of their atomic masses.
Limitations
- Could be valid up to calcium only
- Newlands assumed that only56 elements existed in nature and no more elements would be discovered.
Mendeleev’s Periodic Classification
Mendeleev’s Periodic Law states that the properties of elements are the periodic function of their atomic masses.
Merits of Mendeleev’s Periodic Table
- Mendeleev left some blank spaces for undiscoveredelements.
- Mendeleev predicted the discovery of some elements and named them as eka-boron, ekaaluminium and eka-silicon.
- Noble gases discovered later could be placed without disturbing the existing order.
Limitations of Mendeleev’s Periodic Table
- Position of Hydrogen- Could not assign a correct position to hydrogen as hydrogen resembles alkali metals as well as halogens
- Position of Isotopes- Isotopes are placed in same position though they have different atomic masses
- Separation of chemically similar elements while dissimilar elements are placed in the same group.
Modern Periodic Classification
Modern Periodic Law states that properties of elements are the periodic function of their atomic numbers.
Groups in Modern Periodic Table:
Periods in Modern Periodic Table

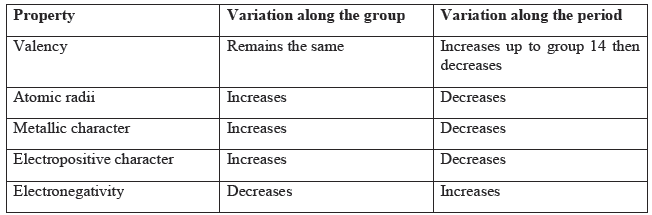
Trends in Modern Periodic Table:

DIAGRAMS
NEWLAND’S OCTAVES

Passage Based Question Periodic Classification of Elements Class 10 Science
Around the year 1800, only 30 elements were known. Dobereiner in 1817 and Newlands in 1866 tried to arrange the then known elements and framed laws which were rejected by the scientists. Even after the rejection of the proposed laws, many scientists continued to search for a pattern that correlated the properties of elements with their atomic masses. The main credit for classifying elements goes to Mendeleev for his most important contribution to the early development of a Periodic table of elements wherein he arranged the elements on the basis of their fundamental property, the atomic mass and also on the similarity of chemical properties. The formulae of their hydrides and oxides were treated as basic criteria for the classifcation of the elements. However, Mendeleev’s classification also had some limitations as it could not assign the position to isotopes. He also left some gaps in the periodic table.
(A) State Mendeleev’s Periodic Law.
(B) Why did Mendeleev leave some gaps in the Periodic table?
(C) If the letter ‘R‘ was used to represent any of the elements in the group, then the hydride and oxide of carbon would respectively be represented as:
(a) RH4, RO
(b) RH4, RO2
(c) RH2, RO2
(d) RH2, RO
(D) Isotopes are:
(a) Atoms of an element with similar chemical properties but different atomic masses.
(b) Atoms of different elements with similar chemical properties but different atomic masses.
(c) Atoms of an element with different chemical properties but same atomic masses.
(d) Atoms of different elements with different chemical properties but same atomic masses.
Answer : (A) Mendeleev’s Periodic Law: It states that the properties of elements are the periodic function of their atomic masses. Explanation: Mendeleev concluded that when elements are arranged in order of increasing atomic masses, similar properties of element repented after a definite gap of atomic masses. This repetition of similar properties after a definite gap of atomic masses is also called periodicity in properties.
(B) Mendeleev left some gaps in the periodic alder for the then undiscovered elements like gallium (Ga), scandium (Sc) and germanium (Ge) when these elements were discovered later on, they were placed in those gaps, without disturbing the existing elements.
(C) (b) RH4, RO2
Analyse the following table given below and answer the questions that follow:
The atomic radii of the element of second period are given below: (Table 114)
(A) Arrange these elements in decreasing order of their atomic radii.
(B) Why does atomic radius decreases as we move from left to right in a period?
(C) Which is not true about the noble gases?
(a) They are non metallic in nature
(b) They exist in atomic form
(c) They are radioactive in nature
(d) Xenon is the most reactive among these
(D) Which of the following is the outermost shell for elements of period 2?
(a) K shell
(b) L shell
(c) M shell
(d) N shell [SQP]
Answer : (A) Elements in decreasing order of their atomic radii (Table 114)
(B) Atomic radius decreases from left to right in a period. This is due to increase in nuclear charge which tends to pull the electrons closer to the nucleus and hence the atomic size decreases.
Atoms of eight elements A, B, C, D, E, F, G and H have the same number of electron shells but different number of electrons in their outermost shells. It was found that elements A and G combine to form an ionic compound. This ionic compound is added in a small amount to almost all vegetables and dishes during cooking. Oxides of elements A and B are basic in nature while those of elements E and F are acidic. The oxide of element D is, however, almost neutral. Based on the above information, answer the following questions:
(A) To which group or period of the periodic table do these elements belong?
(B) What would be the nature of compound formed by a combination of elements B and F?
(C) Which two of these elements could definitely be metals and which would be non metals?
(D) Which one of the eight elements is most likely to be found in gaseous state at room temperature?
Answer : (A) They all belong to the 3rd period.
(B) The compound between B and F will be ionic in nature.
(C) A and B are metals as they form basic oxides, E and F are non metals as they form acidic oxides.
(D) Element H will be found in gaseous state at room temperature as it is the 8th element of the group so it would have 8 electrons in its outermost shell which is the electronic
configuration of a Nobel gas.
Important Questions Periodic Classification of Elements Class 10 Science
Very Short Answer Type Questions
Question. List any two properties of the elements belonging to the first group of modern periodic table.
Answer: (i) They should have valency equal to 1 and form monovalent positive ions.
(ii) They are highly reactive soft metals.
Question. The formula of magnesium oxide is MgO. State the formula of barium nitrate and barium sulphate, if barium belongs to the same group.
Answer: Ba(NO3)2, BaSO4
Question. “Fluorine is more electronegative than iodine”. Give reason in support of this.
Answer: ‘F’ is smaller in size than I, therefore the tendency to gain electrons is more due to more effective nuclear charge.
Question. How does valency vary in a (i) period on going from left to right, (ii) group?
Answer: (i) In a period, valency first increases till the middle and then it decreases.
(ii) In a group, it remains the same.
Question. The electronic configuration of two elements ‘A’ and ‘B’ are 2, 8, 7 and 2, 8, 8, 2, respectively. Write the atomic number of these elements. What will be the formula of the compound formed and the nature of bond between them, when these elements chemically combine together?
Answer: A has atomic number ‘17’, ‘B’ has atomic number ‘20’.
BA2 is the formula of the compound. The bond formed between A and B will be ionic bond.
Question. In the periodic table, how does the tendency of an atom to lose electrons changes on moving from (i) left to right across a period?, (ii) top to bottom in a group?
Answer: (i) It decreases due to increase in effective nuclear charge.
(ii) It increases due to decrease in effective nuclear charge.
Question. What would be nature of oxides formed by the elements on the right hand side of periodic table?
Answer: Acidic
Question. Which is smaller: (i) Na+ or Na, (ii) Cl or Cl– ?
Answer: (i) Na+, (ii) Cl
Question. What is a metalloid? Name any one of them.
Answer: The element which resembles both with metals and non‑metals is called a metalloid, e.g. Boron, Silicon.
Question. Define electropositivity.
Answer: It is defined as measure of tendency to lose electrons. The greater the tendency to lose electrons, more will be electropositivity.
Question. Which has larger atomic radius, K(19) or Ca(20)?
Answer: K(19) is larger than Ca(20).
Question. How does the metallic character of elements changes along a period of the periodic table from left to right and why?
Answer: It decreases due to decrease in atomic size and decrease in tendency to lose electrons.
Question. The atomic radii of first group elements are given below:
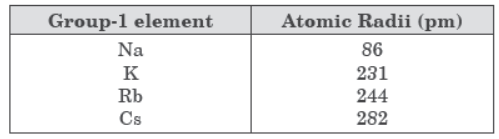
State the reason behind the observed trend in the above elements.
Answer: Atomic radii increases down the group because number of shells go on increasing, effective nuclear charge decreases, distance between nucleus and valence shell increases.
Question. How does metallic character (electropositive character) varies down the group?
Answer: It increases down the group.
Question. Element X forms a chloride with the formula XCl2, which is solid with a high melting point. X would most likely be in the same group of the Periodic Table as (a) Na (b) Mg (c) Al (d) Si.
Answer : As the formula of chloride of element X is XCl2, it is a metal with valency 2 as it has a high melting point and is combining with Cl which is a non-metal having valency 1. Of the given elements, valency of (b) Mg is 2 (Atomic No. 12). Therefore, X will be in the same group as Mg in the Periodic Table.
Question. State the common characteristic of the following elements:Boron, Silicon, Germanium and Arsenic
Answer : Boron, silicon, Germanium and Arsenic elements are metalloids. They possess the properties of both metals and non-metals.
Question. State the Periodic Law on which the Modern Periodic Table is based.
Answer : Periodic Law on which the Modern Periodic Table is based is termed as Modern Periodic Law. According to Modern Periodic Law—The properties of elements are a periodic function of their atomic numbers.
Question. The atomic radii of three elements A, B and C of a periodic table are 186 pm, 104 pm and 143 pm respectively. Giving a reason, arrange these elements in the increasing order of atomic numbers in the period.
Answer : Since atomic size decreases along a period and the atomic number increases. So, the element with smaller radii, has the highest atomic number. Hence, B has the highest atomic number followed by C and A i.e. A < C < B.
Question. Two elements ‘A’ and ‘B’ belong to group 1 and 2 respectively in the same period. Compare them with respect to size of their atoms.
Answer : Size of B will be smaller than the size of A because on moving from left to right in a period, the size of atoms decreases. When we move from left to right in a period, the number of electrons and protons increases. Due to the large positive charge on the nucleus, electrons are pulled more strongly towards the nucleus.
Short Answer Type Questions
Question. How does the valency of an element be determined, if its electronic configuration is known? What will be the valency of an element with atomic number 9?
Answer: Valency is equal to the number of valence electrons when valence electrons are from 1 to 4 or 8 – no. of valence electrons when valence electrons are from 5 to 8.
F(9): 2, 7; It can gain 1 electron to become stable, so its valency = 1.
Question. Three elements ‘X’, ‘Y’ and ‘Z’ having atomic numbers 11, 7 and 6 respectively react with oxygen to form their oxides.
(a) Arrange these oxides in increasing order of their basic nature.
(b) Give reason for your answer.
Answer: X(11): 2, 8, 1; Y(7): 2, 5; Z(6): 2, 4
(a) Y < Z < X
(b) ‘X’ is metallic in nature, therefore it will form basic oxide. ‘Y’ and ‘Z’ are non‑metals will form acidic oxides. ‘Y’ will form more acidic oxide than ‘Z’ because it is more non‑metallic in nature.
Question. Based on the group valency of elements write the molecular formula of the following compounds giving justification for each
(i) Oxides of first group elements
(ii) Halides of group 13 and
(iii) Compound formed when an element A of group 2 combines with element B of group 17
Answer: (i) Group 1 elements have valency equal to 1.

(ii) Group 13 elements have 3 valence electrons, valency equal to 3
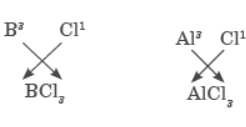

∴ A has valency equal to 2 and B has valency equal to 1.
Question. Two elements ‘P’ and ‘Q’ belong to the same period of the modern periodic table and are in Group 1 and 2 respectively. Compare the following characteristics in tabular form:
(a) The number of valence electrons in their atom.
(b) Their metallic character (c) The size of their atoms
(d) The formulae of their oxides (e) Their tendency to lose electrons
(f) The formula of their chloride
Answer:
Question. Table given below shows a part of the periodic table:
Using this table, explain why
(i) Li and Na are considered as active metals?
(ii) Atomic size of Mg is less than that of Na?
(iii) Fluorine is more reactive than chlorine?
Answer: (i) Li and Na can lose electrons easily due to their large size and hence are more reactive.
(ii) Mg has more effective nuclear charge than Na.
(iii) Fluorine can gain electrons more easily than chlorine, due to smaller atomic size.
Question. How does electronegativity of an element change as we go down a group and across a period? Give reason.
Answer: Electronegativity decreases down the group due to increase in atomic size and decrease in effective nuclear charge.
Electronegativity increases along a period due to decrease in atomic size and increase in effective nuclear charge.
Question. Which is bigger (i) O or F, (ii) N or P and why?
Answer: O is bigger in size than F due to less effective nuclear charge.
P is bigger in size than N due to more number of shells.
Question. What is the position i.e. group number, period number of element, iodine (atomic number 53)? What is the physical state and nature of this element (metal or non metal)?
Answer: I(53): 2, 8, 18, 18, 7
It belongs to group 17, 5th period.
It is a solid. It is a non‑metal.
Question. The elements Be, Mg and Ca are having two electrons in their outermost shells are in periods 2, 3 and 4, respectively of the modern periodic table. Answer the following questions, giving justification in each case.
(i) Write the group to which these elements belong.
(ii) Name the least reactive element.
(iii) Name the element having largest radius.
Answer: (i) They belong to Group 2 because they have 2 valence electrons.
(ii) Be is the least reactive element due to smallest size and least tendency to lose electrons.
(iii) Ca has largest radius because it has the most, four shells (2, 8, 8, 2).
Question. What is meant by ‘group’ in the modern periodic table? How do the following changes occur on moving from top to bottom in a group?
(i) Number of valence electrons (ii) Number of occupied shells
(iii) Size of Atoms (iv) Metallic character of elements
(v) Effective nuclear charge experienced by valence electrons.
Answer: The vertical columns of periodic table are called Groups.
(i) Number of valence electrons remains the same.
(ii) Number of occupied shells goes on increasing.
(iii) Size of atoms increases down the group.
(iv) Metallic character of elements increases down the group.
(v) Effective nuclear charge decreases.
Question. Consider the following and answer the questions that follow:
(i) Amongst A, D and G, which is not electropositive and why?
(ii) Atomic size of H is bigger than B. Why?
(iii) Write the formula of compound formed by the element E and fluorine.
Answer: (i) ‘A’ is not electropositive because it is hydrogen which is considered as a non‑metal.
(ii) ‘H’ has more number of shells than ‘B’, therefore it has bigger atomic size.
(iii) EF2 is the formula of fluoride of ‘E’.
Question. Explain the basic character of oxides of elements down the group and across the period.
Answer: Basic character of oxides increases from top to bottom in a group because metallic character increases down the group due to increase in tendency to lose electrons.
Basic character of oxide decreases along a period from left to right because the atomic size decreases, tendency to lose electrons decreases, metallic character decreases.
Question. The following table shows the position of five elements A, B, C, D and E in the modern periodic table.

Answer the following giving reasons:
(i) Which element is a metal with valency two?
(ii) Which element is least reactive?
(iii) Out of D and E which element has a smaller atomic radius?
Answer: (i) D, As it is on the left side of the table in group 2.
(ii) C, as it is in the group 18/ Noble gas.
(iii) E, as we move from left to right across a period, atomic radius decreases.
Question. Given below are some elements of modern periodic table. Atomic number of elements are given in parenthesis:
A(4), B(9), C(14), D(19), E(20)
(a) Select the element that has one electron in outermost shell. Also write the electronic configuration of this element.
(b) Which two elements amongst there belong to the same group? Give reason for your answer.
(c) Which two elements amongst there belong to the same period? Which one of the two has bigger atomic radius?
Answer: (a) D(19) has one valence electron. Its electronic configuration is 2, 8, 8, 1.
(b) A(4), E(20) belong to same group because they have same number of valence electrons.
(c) A and B belong to same period, A is bigger than ‘B’.
D and E also belong to same period, ‘D’ is bigger than E.
Question. In the following table, are given eight elements A, B, C, D, E, F, G and H (here letters are not the usual symbols of the elements) of the Modern Periodic Table with atomic numbers of the elements in parenthesis.

(i) What is the electronic configuration of F?
(ii) What is the number of valence electrons in the atom F?
(iii) What is the number of shells in the atom F?
(iv) Write the order of size of the atoms of E, F, G and H in decreasing order.
(v) State whether F is a metal or a non‑metal.
(vi) Out of the three elements B, E and F, which one has the biggest atomic size?
Answer: (i) F(12) 2, 8, 2; (ii) 2; (iii) 3;
(iv) H > G > F > E; (v) F is a metal;
(vi) B has biggest atomic size.
Question. Na, Mg and Al are the elements of the 3rd period of the Modern Periodic Table having group number 1, 2 and 13 respectively. Which one of these elements has the (a) highest valency, (b) largest atomic radius, and (c) maximum chemical reactivity? Justify your answer stating the reason for each.
Answer: Given are the three elements Na, Mg and Al belonging to group 1 , 2 and 13 respectively.
The electronic configurations of the three elements are as follows:
(a) The element having the highest valency signifies the maximum number of electrons present in the valence shell of an atom. Hence, as per the given electronic configurations, the element having highest valency is aluminium (Al).
(b) As we move across the period, i.e., from left to right the atomic radius decreases. Therefore the element having the largest atomic radius will be sodium (Na).
(c) The given three elements are metals. So, the chemical reactivity of a metal is determined by its metallic character which is the tendency of an atom to lose its electrons. We know that the metallic character of element decreases across the period, i.e., from left to right. So, the element having highest chemical reactivity is sodium (Na).
Question. Two elements X and Y have atomic numbers 12 and 16 respectively. To which period of the modern periodic table do these two elements belong? What type of bond will be formed between them and why? Also give the chemical formula of the compound formed.
Answer: Electronic configuration of X: 2,8,2, Y: 2,8,6
Both X and Y belong to 3rd period because they have 3 shells.
Ionic bond will be formed.
Reason: X will lose 2 electrons and Y will gain 2 electrons to complete their octet and become stable.
Formula is (X2+){..Y2-}
Question. The atomic number of Na and Mg is 11 and 12 respectively and they belong to same period.
(a) Which one would have smaller atomic size?
(b) Which one would be more electropositive?
(c) To which group would each one belongs?
Answer: (a) Magnesium has smaller size than Na.
(b) Na is more electropositive than Mg.
(c) Na belongs to Group 1, Mg belongs to Group 2.
Question. Write the names given to the vertical columns and horizontal rows in the Modern Periodic Table. How does the metallic character of elements vary on moving down a vertical column? How does the size of atomic radius vary on moving left to right in a horizontal row? Give reason in support of your answer in the above two cases.
Answer: In the Modern Periodic Table, there are 18 vertical columns known as Groups and 7 horizontal rows known as Periods.
As we move down the group, the electrostatic attraction between the nucleus and the outer‑most electron decreases due to increase in the distance between them. This happens because, on moving down the group, a new shell is added. So the valence electrons can be easily lost by the element. As we know, metallic character is characterised by the ease of loss of electrons, thereby, metallic character increases on moving down the group in the Modern Periodic Table.
When we move across a period, the number of electrons in the same shell increases. This leads to greater electrostatic attraction between the nucleus and the outer‑most electron. This increased attraction pulls the outer‑most electron closer to the nucleus, thereby decreasing the atomic size.
Question. The electronic configuration of an element ‘X’ is 2,8,6. To which group and period of the modern periodic table does ‘X’ belong .State it valency and justify your answer in each case.
Answer: X – 2, 8, 6
(a) Since ‘X’ has three energy shells and period number of an element is equal to the number of energy shells. So, X belongs to 3rd period.
(b) X has 6 valence electrons so, it belongs to group 16.
(c) Valency will be 2. To acquire noble gas configuration it will gain 2 electrons.
Question. Write the number of periods and groups in the Modern Periodic Table. How does the metallic character of elements vary on moving (i) from left to right in a period, and (ii) down a group? Give reason to justify your answer.
Answer: In the Modern Periodic Table, there are 18 vertical columns known as Groups and 7 horizontal rows known as Periods.
Metallic character: It is defined as the tendency of an atom to lose electrons.
Across the period i.e., from left to right: Metallic character decreases.
Down the group i.e., from top to bottom: Metallic character increases.
Reason: Across the period, the effective nuclear charge increases, thus decreasing its atomic radius.
This favours the increase of electronegativity and therefore the tendency to lose electrons is less. This accounts for the decrease in the metallic character along the period.
But as we move down the group the number of shells keep on increasing and therefore the atomic size increases. This means that the electronegativity decreases. This enhances the ability to lose electrons and therefore the metallic character increases down the group.
Question. How is possible valency of element determined with the help of electronic configuration of its atom?
Determine the valency of ‘X’ whose atomic number is 15.
Answer: Valency is equal to number of valence electrons or 8–valence electrons. X has electronic configuration 2, 8, 5.
Its valency is equal to 3 because it can gain 3 electrons to become stable.
Question. Three elements A, B and C have 3, 4 and 2 electrons respectively in their outermost shell. Give the group number to which they belong in the modern periodic table. Also, give their valencies.
Answer : The group number for elements can be identified as 10 + number of valence electrons. Element A with 3 valence electrons will show valency 3 and should belong to group 13. The elements of group 13 are B, Al, Ga, In or Tl. Element B has 4 valence electrons, so it must be from group 14(10 + 4). The element B can be C, Si, Ge, Sn or Pb. Similarly, element C has two valence electrons; hence, it must be from group 2. So element C can be Be, Mg, Ca, Sr, Ba or Ra.
Question. Two elements X and Y have atomic numbers 12 and 16 respectively. To which period of the modern periodic table do these two elements belong? What type of bond will be formed between them and why? Also give the chemical formula of the compound formed.
Answer : Electronic configuration of X: 2,8,2, Y: 2,8,6 Both X and Y belong to 3rd period. Ionic bond will be formed. Reason: X will lose 2 electrons and Y will gain 2 electrons to complete their octet and become stable. Formula is XY.
Question. Answer the following:
(A) Name any three halogens.
(B) Mention the group to which they belong and their valency.
(C) What type of compounds will they form with elements of group 1?
Answer : (A) Three halogens are Fluorine, Chlorine and Bromine.
(B) They belong to the Halogen group or Group 17 of the periodic table. Their valency is 1.
(C) All the alkali metals react vigorously with halogens to produce salts, the most industrially important of which are NaCl and KCl. 2Na(s) + Cl2(g) → 2NaCl(s)
Question. Compare the radii of two species X and Y. Give reasons for your answer.
• X has 12 protons and 12 electrons
• Y has 12 protons and 10 electrons
Answer : Here, X has the same number of electrons and protons, therefore, it is a neutral atom, whereas Y contains 12 protons and 10 electrons, so there are 2 protons extra, giving Y a charge of +2. The electronic configurations of the two species can be expressed as:
The atomic size of a cation is always smaller than a neutral atom containing the same number of protons. This is because cation has less number of electrons, due to which the nuclear attraction is more on electrons. Hence, the size of a cation is smaller than that of the neutral atom. Hence, atomic radius of Y is smaller than that of X.
Question. Write the formula of the product formed when the element A (atomic number 19) combines with the element B (atomic number 17). Draw its electronic dot structure. What is the nature of the band formed?
Answer : The electronic configuration of element A with atomic number 19 would be 2, 8, 8, 1. Since it has only one valence electron, it must be a metal that is potassium. The electronic configuration of element B would be 2, 8, 7 with 7 valence electrons. So, it must be a non-metal that is chlorine. A metal and a non-metal usually combine through ionic bond because metals have a tendency to lose electrons and form cations, whereas non-metals can accept electrons to form anions.
Potassium and chlorine will form potassium chloride (KCl).
The electron dot structure of KCl is as given below:
Question. Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Answer : Electronic configurations of the two elements are:
Nitrogen is more electronegative than phosphorus. On moving down a group, the number of shells increases. Therefore, the valence electrons move away from the nucleus and the effective nuclear charge decreases. This causes the decrease in the tendency to attract electrons and hence electronegativity decreases.
Question. Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Answer : Electronic configurations of the two elements are:
Element K L M
Nitrogen (7) 2 5
Phosphorus (15) 2 8 5
Both these elements have 5 electrons in their respective valence shell and thus have a tendency to gain 3 more electrons to form negative ions by completing their respective octet. Nitrogen is more electronegative than phosphorus. On moving down a group, the number of shells increases. Therefore, the valence electrons move away from the nucleus and the effective nuclear charge decreases.
This causes the decrease in the tendency to attract electrons and hence electro- negativity decreases.
Question. Answer the following: (A) Why do elements in a group show similar property?
(B) An element M is in the third group of the periodic table. Write the formulae of its chloride and oxide.
Answer : (A) All the elements present in a group have same electronic configuration of the atoms. The physical and chemical properties of elements depend on the number of valence electrons. Elements present in the same group have the same number of valence electrons. Therefore, elements present in the same group have similar physical and chemical properties.
Question. Identify and name the metals out of the following elements whose electronic configurations are given below:
(a) 2, 8, 2
(b) 2, 8, 1
(c) 2, 8, 7
(d) 2, 1
Answer : Elements having 1 to 3 valence electrons are usually metals, whereas elements with 4 or more valence electrons are usually non-metals or metalloids.
The following elements whose electronic configurations are given below:
Thus, elements (a), (b) and (d) are metals, while (c) is a non-metal.
Question. An element ‘X’ belong to 3rd period and group 13 of the Modern Periodic Table.
(A) Determine the valence electrons and the valency of ‘X’.
(B) Molecular formula of the compound formed when ‘X’ reacts with an element ‘Y’ (atomic number = 8).
(C) Write the name and formula of the compound formed when ‘X’ combines with chlorine.
Answer : As the element ‘X’ belongs to the 3rd period and group 13 of the modern periodic table,
its electronic configuration is: 2, 8, 3 (K-shell: 2,L-shell: 8, M-shell: 3) and atomic number is 13.
(A) The valence electrons of ‘X’ are 3 and it is a metal having valency = 3 (valency = no. of valence electrons).
(B) As atomic number of ‘Y’ is 8, it’s electronic configuration is 2, 6. It is a non-metal having valency 2.
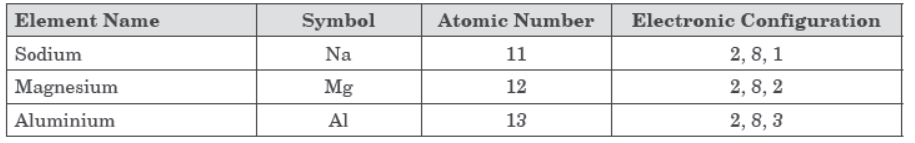
Therefore, when ‘X’ (a metal having valency 3) reacts with ‘Y’ (a non-metal having valency 2), the formula of compound formed will be X2Y3 as shown below.
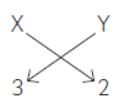
(C) Atomic number of chlorine = 17 and its
electronic configuration is 2, 8, 7. Therefore, its valency = 1.
The formula of compound formed when ‘X’ combines with Chlorine will be XCl3.
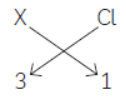
As ‘X’ is aluminium (atomic number=13), formula of its chloride is AlCl3 and its name is aluminium chloride.
Question. An element X has mass number 35 and the number of neutrons is 18. Identify the group number and period of X.
Answer : Mass no. = Sum of protons + Sum of neutrons
35 = Sum of protons + 18
Sum of protons = 35 – 18
Sum of protons = 17
Hence, we can say that the atomic number of element X is 17.
Its electronic configuration will be 2, 8, 7.
Thus it is clear that element X belongs to the 3rd Period and the 17th Group.
Question. An element ‘X’ has mass number 35 and number of neutrons 18. Write atomic number and electronic configuration of ‘X’. Also write group number, period number and valency of ‘X’.
Answer : mass Number = 95
no of neutrons = 18
atoms number of x = 17
electronic configuration = 3,8,7
Group Number : 17
Period Number 3
Question. Out of lithium and potassium, which one has stronger metallic character and why?
Answer : On going down in a group of the periodic table,the metallic character of elements increases.
Therefore, potassium has stronger metallic character than Lithium.
The reason behind is that as we go down in a group of the periodic table, one more electron shell is added. As a result, the size of the atom increases. The valence electrons move away from the nucleus at regular intervals. The holding capacity of the nucleus on valence electrons decreases. Due to this the atom can lose valence electrons more easily to form
positive ions, and hence the metallic character increases.
Long Answer Type Questions
Question. (a) How is valency of an element determined if its electronic configuration is known? Determine the valency of an element of atomic no. 9
(b) Given below are some elements of modern periodic table. Atomic number of elements are given in parentheses.
A (4), B (9), C (14), D (19), E (20)
(i) With the help of electronic configuration, find out which ore of the above elements will have one
electron in its outermost shell.
(ii) Which two elements belong to the same group? Give reasons for your answer.
(iii) Which one of the above element belonging to the fourth period has bigger atomic radius and why?
Answer: (a) Valency is equal to number of valence electrons or 8— number of valence electrons.
F (9): 2,7
Its valency is equal 1 because it will become stable on gaining one electron.
(b) (i) A (4): 2,2 D (19): 2,8,8,1
B (9): 2,7 E (20): 2,8,8,2
C (14): 2,8,4
‘D’ has one valence electron.
(ii) ‘A’ and ‘E’ belong to same group because they have same number of valence electrons.
(iii) ‘D’ has larger atomic radius than ‘E’ because it has 19 protons which attract 19 electrons which is there less strongly than 20 protons can attract 20 electrons as in E.
Question. Explain giving justification the trends in the following properties of elements, on moving from left to right in a period, in the Modern Periodic Table.
(a) Variation of valency (b) Change of atomic radius
(c) Metallic to non‑metallic character (d) Electronegative character
(e) Nature of oxides
Answer: (a) Valency increases from left to right till middle, then decreases because valence electrons goes on increasing.
(b) Atomic radius decreases due to increase in effective nuclear change.
(c) Metallic character decreases, non‑metallic character increases due to increase in tendency to gain electrons as atomic size decreases, effective nuclear change increases.
(d) Electronegative character increases due to increase in effective nuclear change.
(e) Acidic character of oxides increases, basic character of oxides decreases because metallic character decreases and non‑metallic character increases.
Question. Define atomic size. Give its unit of measurement. In the modern periodic table what trend is observed in the atomic radius in a group and a period and why is it so?
Answer: Atomic size is the distance between centre of nucleus and valence shell.
Its unit of measurement is picometre (10–12 m) denoted by pm.
Atomic radius increases down the group due to increase in number of shells and decrease in effective nuclear charge.
Atomic radius decreases along a period from left to right because effective nuclear charge increases but number of shells remain the same.
Question. The atomic radius of element of second period are given below:

(i) Arrange these elements in decreasing order of atomic radius.
(ii) Are the elements now arranged in the pattern of period in the periodic table?
(iii) Name the element which has (a) largest (b) smallest atomic size.
(iv) From the above data, infer how the atomic size or radius of elements changes as we go from left to right in a period.
(v) Name one metal, one non‑metal and a metalloid from these elements.
(vi) Why does atomic radius decrease as we move from left to right in a period?
Answer: (i) Li Be B C N O F
152 111 88 77 74 66 64
(ii) Yes, the elements are arranged in the pattern of a period (2nd period in the periodic table).
(iii) (a) Li is largest in size.
(b) F is smallest in size.
(iv) Atomic radius decreases from left to right in a period.
(v) Metal–Li/Be, Non‑Metal‑C/N/O/F, Metalloid‑Boron.
(vi) Atomic size decreases as we move from left to right in a period because effective nuclear charge increases as one electron and one proton is added successively and number of shells remains the same.
Question. (a) List any three observations which posed a challange to Mendeleev’s periodic table.
(b) How does the mettallic character of elements vary on moving from
(i) Left to right in a period.
(ii) From top to bottom in a group of the Modern periodic table? Give reason for your answer.
Answer: (a) (i) Increasing order of atomic mass could not be followed.
(ii) Isotopes cannot occupy different positions as they have same chemical properties but different atomic mass.
(iii) Position of hydrogen was not justified.
(b) (i) Metallic character decreases from left to right in a period because tendency to lose electrons decreases as effective nuclear charge increases.
(ii) Metallic character increases down the group from top to bottom because tendency to lose electron increases due to increase in atomic size and decrease in effective nuclear charge.
Question. Why is atomic number considered to be a more appropriate parameter than atomic mass for the classification of elements in a periodic table? How does the metallic character of elements vary as we move
(i) from left to right in a period, and
(ii) top to bottom in a group in the modern periodic table? Give reasons to justify your answers.
Answer : When elements were arranged on the basis of increasing atomic number, prediction of their properties could be made with more precision. Moreover, many anomalies of the Mendeleev’s periodic table, such as position of isotopes, position of elements such as Cobalt and Nickel, could be explained.
(1) The metallic character decreases as we move from left to right in a period. Because when we move from left to right in a period, the effective nuclear charge acting on the valence shell electrons increases and therefore the tendency to lose electrons also decreases.
(2) The metallic character increases as we move from top to bottom in a group because when we go down a group, the effective nuclear charge experienced by valence electrons decreases since distance of valence electrons from the nucleus increases.
(i) Position of isotopes : All the isotopes of an element have the same atomic number. Therefore, They can be place at one place is the same group of the periodic table. e.q. C-12, C-14 are placed is group 14.
(ii) Anomalous position of some pairs of elements.
In mendeleev’s periodic table, cobalt with slightly higher atomic mass (58.93μ) war placed before nickel with slightly lower atomic mass (58.71μ). But the atomic number of Co is less than that of Ni, as Modern Periodic Law is based on atomic number so this anomaly was explained
For example: In third period: Mg is smaller than Na because on moving form left to right in a period, atomic radius of element decreases. This happens because the number of protons and electrons is the atoms increases. Due to a large positive charge on the nucleus, electrons are pulled close to the nucleus and size of atom decreases and metallic character decreases.
For example: In the first group Na (Sodium) atom has three electron shells K, L, and M and K atom has four electrons shells K, L, M and N and K (Potassium) atom is bigger than Na because when we go from top to bottom in a group, a new shell of electrons is added to the atom, due to which the size of atoms increases and metallic character also increases.
Question. Write the electronic configurations of two elements P (atomic number 17) and Q (atomic number 19) and determine their group numbers and period numbers in the Modern Periodic Table.
Answer : The electronic configuration of elements P and Q is written below:
Position of P and Q in the Modern Periodic Table:
P: Group number = 17 ; Period number = 3
Q: Group number = 1 ; Period number = 4
Question. An element ‘X’ with electronic configuration (2, 8, 2) combines separately with two radicals, (NO3)– and (SO4)2–.
(A) Is ‘X’ a metal or a non-metal ? Write the nature of its oxide.
(B) Write the formula of the compounds of ‘X’ formed by the combination of these radicals. Are these compounds covalent or electrovalent ?
Answer : (A) ‘X’ is a metal as it has 2 valence electrons. Its oxide is basic in nature.
(B) Valency of X is 2. The formula of compounds formed by X with (NO3)– and (SO4)2– :
a. X(NO3)2
b. XSO4
These compounds are ionic (electrovalent) as these are formed by transfer of electrons.
Question. The electronic configuration of an element is 2, 8, 7.
(A) What is the group number and period number of this element in the Modern Periodic Table?
(B) Is this element a metal or a non-metal?
Give reason to justify your answer.
Answer : (A) Its electronic configuration is 2, 8, 7. Hence its atomic number is 17 and this element is chlorine. It belongs to 17 group and third period in the modern periodic table
(B) This element is a non-metal because it gains the electron to complete their octet.
Question. An element ‘M’ has atomic number 12.
(A) Write its electronic configuration and valency.
(B) Is ‘M’ a metal or a non-metal? Give reason in support of your answer.
(C) Write the formula and nature (acidic/ basic) of the oxide of M.
Answer : Atomic number of element ‘M’ = 12
(A) Electronic configuration of M: (2, 8, 2), i.e., k-shell = 2, L-shell = 8, M-shell = 2 electrons.
Valency of M = 2
(B) M is a metal as it is electropositive, since it has the tendency of losing electrons,thereby forming positive ion.
(C) Formula of the oxide of M: MO
Nature of oxide: Basic
Question. How does the atomic radius of the elements change on going:
(A) from left to right in a period, and
(B) down a group in the Modern Periodic Table? (Give reasons) in support of your answer.
Answer : (A) The atomic radius decreases on moving from left to right in a period in the Modern Periodic Table as the number of electrons and protons increases. Due to the large positive charge on the nucleus, electrons are pulled more strongly towards the nucleus.
(B) The atomic radius increases on moving from top to bottom in a group, as new shells are being added, which increases the distance between the outermost electrons and the
nucleus. So, although the nuclear charge increas, the atomic radius also increases.
Question. What is meant by groups and periods in the Modern Periodic Table? Two elements ‘A’ and ‘B’ belong to group 1 and 2 respectively and are in the same period. How do the following properties of ‘A’ and ‘B’ vary?
(A) Atomic size
(B) Metallic character
(C) Valencies in forming oxides
(D) Formula of their chlorides
Answer : Groups are the vertical Colums Periods are the horizontal rows.
(A) Atomic size: decreases as we go from left to right in a period hence atom of element A is bigger than B.
(B) Metallic Character: A is more metallic than B since metallic character decreases as we go along a period.
(C) Valancies in forming oxides: Valency of group 1 is 1 and valency of group 2 is 2.
(D) Formula of their chloride: ACl, BCl2.
Question. Write the names given to the vertical columns and horizontal rows in the Modern Periodic Table. How does the metallic character of elements vary on moving down a vertical column? How does the size of atomic radius vary on moving left to right in a horizontal row? Give reason in support of your answer in the above two cases.
Answer : The vertical columns in the Modern Periodic Table are known as ‘Groups’ and horizontal rows are known as ‘Periods’. The metallic character increases as we move from top to bottom in a group because the electropositive character of elements increases as the tendency of an atom to lose electrons increases. On moving from left to right in a period, the atomic radius decreases as the number of electrons and protons increases. Due to the large positive charge on the nucleus, electrons are pulled more strongly towards the nucleus.
Question. Do you know there are 118 elements known at present. It is very difficult to study the properties of all these elements. Different scientists started looking for some pattern in this properties.
Dobereiner arranged elements with lame properites into triads. Newlands assumed that when elements are arranged in the increasing order of there atomic mass. The properties of every eighth elements are similar to one. Mendeleev stated that properties of elements are periodic function of their atomic mass. Mosley gave the Modern Periodic Table and it sates that properties of elements are peridic function of their atomic numbers. Solve the questions on the basis of your understanding of the above paragraph and the related studied concepts.
(A) Compare and contrast the arrangement of elements in Mendeleev’s Periodic Table and Modern Periodic Table.
(B) Hygrogen occupies a unique position in modern periodic table. Thus it occupies a unique position in modern periodic table. Justify the statement.
Answer : (A)
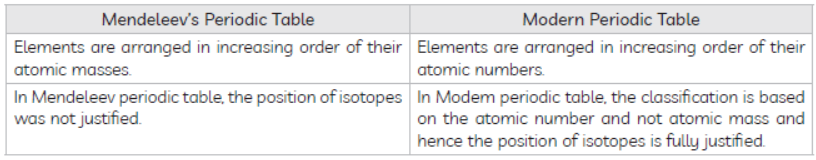
(B) Hyrogen shows similar properties like alkali metals as well as hydrogen.
Question. An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reason:
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q.
Answer : In order to answer this question, let us write the electronic configuration of the given elements:
Element P (Atomic Number = 20): 2, 8, 8, 2
Element Q (Atomic Number = 17): 2, 8, 7
Position of P: Period No. 4 and Group No. 2
Position of Q: Period No. 3, Group No. 17
P is a metal having valency of 2 and Q is a nonmetal having valency of 1, therefore formula of compound formed by the reaction between P and Q will be PQ2.
Question. An element ‘X’ belongs to third period and second group of the Modern Periodic Table.
(a) Write its electronic configuration.
(b) Is it a metal or non-metal? Why? (c) Write the formula of the compound formed when ‘X’ reacts with an element
(i) Y of electronic configuration 2, 6 and
(ii) Z with electronic configuration 2, 8, 2.
Answer : Element situate in 3rd period means it has 3 shells and in second group means it has 2 electrons in outer most shell. Electronic configuration.
(A) X = 2, 8, 2
(B) Because it is situated on left of periodic table therefore it is a metal.
(C) (i) Compound XY
(ii) Compound XZ2
Question. State the main aim of classifying elements.
Which is the more fundamental property of elements that is used in the development of Modern Periodic Table? Name and state the law based on this fundamental property.
On which side of the periodic table one can find metals, non-metals and metalloids?
Answer : (1) Main objective for classification of elements is the systemic study of properties of known element and can guess for future elements.
(2) Basic Property to classify elements is:Atomic number.
(3) Modern Periodic Law: Atomic number is the periodic function for properties of elements.
(4) Metals are situated on left of periodic table.
(5) Non- metals are situated on right of periodic table.
(6) Metalloids are situated on border of metals and non – metals and are placed diagonally.
Question. Four elements P, Q, R, S have atomic number 12, 13, 14 and 15 respectively. Answer the following questions giving reasons:
(i) What is the valency of Q?
(ii) Classify these elements as metals and non-metals.
(iii) Which of these elements will form the most basic oxide?
Answer :
| Element | Atomic Number | Valency |
| P | 12 | 2 |
| Q | 13 | 3 |
| R | 14 | 4 |
| S | 15 | 3.5 |
(i) Valency of Q is 3
(ii) Metals are P & Q
Non-metals S
Metalloids R
As we know all metals are basic in nature and produce basic Oxide.
Both P and Q are metals and P is more reactive metal than Q as P has to lose only two electrons whereas Q has to lose 3 electrons to acquire metallic character hence P is will form the most basic oxide.
Question. An element has electronic configuration 2, 8,7.
(A) To which group and period of the Modern Periodic Table does it belong?
(B) What is the atomic number of this element?
Is it metallic or non-metallic and why?
(C) Identify the element and name an element chemically similar to this element.
Answer : Electronic configuration 2, 8, 7 (total electrons are 17)
(A) It belong to 17th group and 3rd period of periodic table.
(B) Atomic Number of this element is 17, it is non-metallic because it gains electrons to complete its octet.
(C) Element is chlorine, Related elements are Bromine, Iodine, etc.
Question. Calcium is an element with atomic number 20. Stating reason answer each of the following questions:
(A) Is calcium a metal or non-metal?
(B) Will its atomic radius be larger or smaller than that of potassium with atomic number 19?
(C) Write the formula of its oxide.
Answer : (A) Calcium is a metal because it has two electrons in its outermost shell, hence it loses electron easily.
(B) The atomic radius of calcium is smaller than that of potassium because atomic size decreases along the period due to increase in nuclear charge between the electrons.
(C) CaO is the formula of oxide of calcium.
Question. An element ‘M with electronic configuration (2, 8, 2) combines separately with (NO3)–, (SO4)2- and (PO4)3- radicals. Write the formula of the three compounds so formed.
To which group and period of the Modern Periodic Table does the elements ‘M’ belong?
Will ‘M’ form covalent or ionic compounds? Give reason to justify your answer.
Answer : The electronic configuration is 2, 8, 2, Hence the element ‘M‘ is Magnesium (Mg).
It belongs to 2nd group and 3rd period of the modern periodic table and its valency is 2.
The formula of compounds so formed are:
(i) Mg (NO3)2
(ii) Mg SO4
(iii) Mg3 (PO4)2
It will from ionic compound because it has 2 electrons in its outermost shell, so it can loose 2 electrons easily and forms ionic compound.
Question. Compare the following elements as per the characteristics given in the brackets:
(A) Lithium and Nitrogen (Atomic radii)
(B) Potassium and Chlorine (Electronegativity)
(C) Magnesium and Calcium (Valency)
Give reason for your answer in each case.
Answer : (a) Lithium has larger atomic radius compared to nitrogen,
(b) Chlorine is more electronegative than potassium.
(c) Magnesium and Calcium have same valency.
Question. Taking the example of an element of atomic number 16, explain how the electronic configuration of the atom of an element relates to its position in the modern periodic table and how valency of an element is calculated on the basis of its atomic number.
Answer : Electronic configuration of element with atomic no. 16 is 2,8,6.
Since it has 3 shells, the period no. will be 3.
Since the no. of valence electrons is 6, the group no. will be 10 + 6 = 16.
Valency of the element will be 8- valence electrons ie 8 – 6 = 2.
Question. Two elements ‘P’ and ‘Q’ belong to the same period of the modern periodic table and are in Group-1 and Group-2 respectively. Compare their following characteristics in tabular form:
(A) The number of electrons in their atoms
(B) The sizes of their atoms
(C) Their metallic characters
(D) Their tendencies to lose electrons
(E) The formula of their oxides
(F) The formula of their chlorides
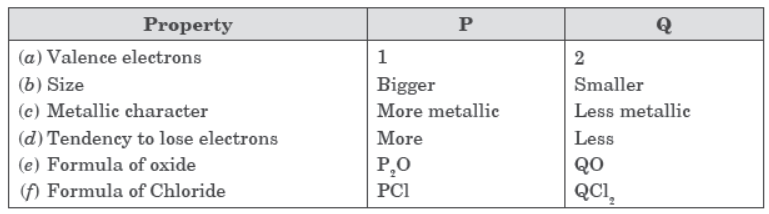
Answer : Property P Q
(A) No. of electrons in the 3 4
atom 11 12
19 20
(B) Size of the atom Bigger Smaller
(C) Metallic character More Less metallic metallic
(D) Tendency to lose More Less electrons
(E) Formula of oxides P2O QO
(F) Formula of chlorides PCl QCl2



