Solutions Class 12 Chemistry Important Questions
Please refer to Solutions Class 12 Chemistry Important Questions with answers below. These solved questions for Chapter 2 Solutions in NCERT Book for Class 12 Chemistry have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 12 Chemistry for all chapters in your textbooks.
Important Questions Class 12 Chemistry Chapter 2 Solutions
All Solutions Class 12 Chemistry Important Questions provided below have been prepared by expert teachers of Standard 12 Chemistry. Please learn them and let us know if you have any questions.
Very Short Answer Questions :
Question. Define the following term :
Molarity
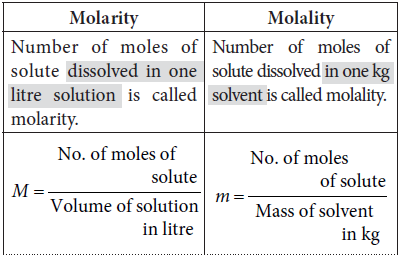
Answer : Number of moles of solute dissolved in one litre solution is called molarity. It is denoted by M.
M = Number of moles of solute/Volume of solution in litre
Question. How is it that alcohol and water are miscible in all proportions ?
Answer : Both alcohol and water are polar in nature hence, they are miscible in all proportions. Water and ethanol molecules attract each other because of the formation of H-bonds. This property also makes them miscible.

Question. State the following :
Henry’s law about partial pressure of a gas in a mixture.
Answer : Henry’s law states that, the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
p = KH⋅x where, KH = Henry’s law constant. Different gases have different KH values at the same temperature.
Question. What is meant by molality of a solution?
Answer : Molality of a solution can be defined as the number of moles of solute dissolved in one kg solvent. It is denoted by m.
m = Number of moles of solute/Mass of solvent in kg = n2/W1
Question. State the main advantage of molality over molarity as the unit of concentration.
Answer : Molality is independent of temperature, whereas molarity is a function of temperature.
Question. How is the vapour pressure of a solvent affected when a non-volatile solute is dissolved in it?
Answer : When a non-volatile solute is added to a solvent, the vapour pressure of the solvent (above the resulting solution) is lower than the vapour pressure above the pure solvent.
Question. Define the following term :
Mole fraction
Answer : Mole fraction is the ratio of number of moles of solute or solvent and total number of moles of solution. It is denoted by x.
xsolute = n2/n1+n2, xsolvent = n1/n1+n2
Question. Gas (A) is more soluble in water than gas (B) at the same temperature. Which one of the two gases will have the higher value of KH (Henry’s constant) and why?
Answer : According to Henry’s law, the solubility of a gas is inversely proportional to the Henry’s law constant (KH) for that gas. Hence, gas (B) being less soluble, would have a higher KH value.
Question. Define the following term :
Osmotic pressure
Answer : Osmotic pressure is the extra pressure which is applied on the solution to just prevent the flow of solvent into the solution through a semi-permeable membrane.
Short Answer Questions :
Question. Assuming complete dissociation, calculate the expected freezing point of a solution prepared by dissolving 6.00 g of Glauber’s salt, Na2SO4⋅10H2O in 0.100 kg of water.
(Kf for water = 1.86 K kg mol–1, atomic masses :
Na = 23, S = 32, O = 16, H = 1)
Answer :

Question. What is meant by negative deviation from Raoult’s law? Give an exmaple. What is the sign of ΔmixH for negative deviation?
Answer : Negative deviation : For non-ideal solution, if the vapour pressure is lower, then it is said to exhibit negative deviation. A—B interactions are stronger than A—A and B—B interactions. Due to this, vapour pressure decreases which results in negative deviation. In negative deviation, intermolecular force increases, volume decreases, vapour pressure decreases and heat is released. Therefore, ΔHmix = ve, ΔVmix = – ve Example, phenol + aniline and chloroform + acetone show negative deviation.

Question. Non-ideal solutions exhibit either positive or negative deviations from Raoult’s law. What are these deviations and why are they caused?
Explain with one example for each type.
Answer : Positive deviation : For non-ideal solutions, if the vapour pressure is higher, then it is said to exhibit positive deviation. A—B interactions are weaker than A—A or B—B interactions. Due to this, vapour pressure increases which results in positive deviation. In positive deviation, intermolecular force decreases, volume increases, vapour pressures increases. enthalpy increases. Therefore, ΔHmix = +ve, ΔVmix = + ve. e.g., ethanol + acetone and carbon disulphide + acetone show positive deviation.

Negative deviation : For non-ideal solution, if the vapour pressure is lower, then it is said to exhibit negative deviation. A—B interactions are stronger than A—A and B—B interactions. Due to this, vapour pressure decreases which results in negative deviation. In negative deviation, intermolecular force increases, volume decreases, vapour pressure decreases and heat is released. Therefore, ΔHmix = ve, ΔVmix = – ve Example, phenol + aniline and chloroform + acetone show negative deviation.
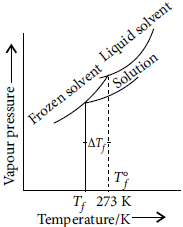
Question. An aqueous solution of sodium chloride freezes below 273 K. Explain the lowering in freezing point of water with the help of a suitable diagram.
Answer : When a non-volatile solute is added to a solvent, the freezing point of the solution is always lower than that of pure solvent as the vapour pressure of the solvent decreases in the presence of non-volatile solute.
Plot for the lowering in freezing point of water when NaCl is added to it is shown as :
Question. A 5% solution (by mass) of cane-sugar in water has freezing point of 271 K. Calculate the freezing point of 5% solution (by mass) of glucose in water if the freezing point of pure water is 273.15 K.
[Molecular masses : Glucose C6H12O6 : 180 amu; Cane-sugar C12H22O11 : 342 amu]
Answer :

Question. A 1.00 molal aqueous solution of trichloroacetic acid (CCl3COOH) is heated to its boiling point. The solution has the boiling point of 100.18°C. Determine the van’t Hoff factor for trichloroacetic acid.
(Kb for water = 0.512 K kg mol–1)
Answer :


Question. Some ethylene glycol, HOCH2CH2OH, is added to your car’s cooling system along with 5 kg of water. If the freezing point of water-glycol solution is –15.0°C, what is the boiling point of the solution
(Kb = 0.52 K kg mol–1 and Kf = 1.86 K kg mol–1 for water)
Answer :

Question. 1.00 g of a non-electrolyte solute dissolved in 50 g of benzene lowered the freezing point of benzene by 0.40 K. The freezing point depression constant of benzene is 5.12 K kg mol–1. Find the molar mass of the solute.
Answer :
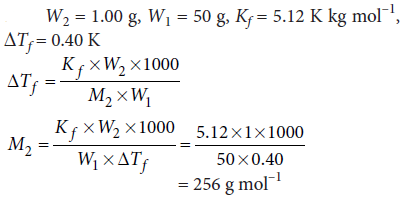
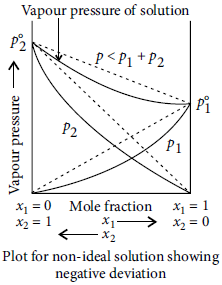
Question. What type of deviation is shown by a mixture of ethanol and acetone? Give reason.
Answer : A mixture of ethanol and acetone shows positive deviation from Raoult’s law. Pure ethanol possesses hydrogen bonding. Introduction of acetone between the molecules of ethanol results in breaking of some of these hydrogen bonds. Due to weakening of interactions, the solution shows positive deviation from Raoult’s law.
Question. What is meant by positive and negative deviations from Raoult’s law and how is the sign of ΔmixH related to positive and negative deviations from Raoult’s law?
Answer : Positive deviation : For non-ideal solutions, if the vapour pressure is higher, then it is said to exhibit positive deviation. A—B interactions are weaker than A—A or B—B interactions. Due to this, vapour pressure increases which results in positive deviation. In positive deviation, intermolecular force decreases, volume increases, vapour pressures increases. enthalpy increases. Therefore, ΔHmix = +ve, ΔVmix = + ve. e.g., ethanol + acetone and carbon disulphide + acetone show positive deviation.

Negative deviation : For non-ideal solution, if the vapour pressure is lower, then it is said to exhibit negative deviation. A—B interactions are stronger than A—A and B—B interactions. Due to this, vapour pressure decreases which results in negative deviation. In negative deviation, intermolecular force increases, volume decreases, vapour pressure decreases and heat is released. Therefore, ΔHmix = ve, ΔVmix = – ve Example, phenol + aniline and chloroform + acetone show negative deviation.

Question. Calculate the boiling point of solution when 4 g of MgSO4 (M = 120 g mol–1) was dissolved in 100 g of water, assuming MgSO4 undergoes complete ionization.
(Kb for water = 0.52 K kg mol–1)
Answer :

Question. x g of a non-electrolytic compound (molar mass = 200) is dissolved in 1.0 L of 0.05 M NaCl aqueous solution. The osmotic pressure of this solution is found to be 4.92 atm at 27°C. Calculate the value of x. Assume complete dissociation of NaCl and ideal behaviour of the solution. (R = 0.082 L atm mol–1 K–1)
Answer :


Question. What concentration of nitrogen should be present in a glass of water at room temperature?
Assume a temperature of 25°C, a total pressure of 1 atmosphere and mole fraction of nitrogen in air of 0.78. [KH for nitrogen = 8.42 × 10–7 M/mm Hg]
Answer : T = 25°C + 273 = 298 K
Total pressure (Ptotal) = 1 atm
pN2 = mol fraction of N2 in air × Ptotal = 0.78 × 1 atm = 0.78 atm = 0.78 × 760 mm = 592.8 mm
As KH is in the units of M(mm)–1, Henry’s law is applied in the form :
Conc. in solution = KH pN2 = 8.42 × 10–7 M (mm)–1 × 592.8 mm
= 4.99 × 10–4 M
Question. Differentiate between molarity and molality of a solution. Explain how molarity value of a solution can be converted into its molality?
Answer :
If MB is the molar mass of solute, d is the density of solution then molarity (M) value of a solution can be converted into its molality (m) by using the following formula,
m = 1000 x M/(1000 X d) – (M x MB )
Question. Define azeotropes. What type of azeotrope is formed by positive deviation from Raoult’s law? Give an example.
Answer : Azeotropes are the binary mixtures of solutions that have the same composition in liquid and vapour phases and that have constant boiling points.
It is not possible to separate the components of azeotropes by fractional distillation.
Question. What type of intermolecular attraction exists in each of the following pairs of compounds :
(i) n-hexane and n-octane
(ii) methanol and acetone
Answer : (i) n-Hexane and n-octane : London dispersion forces as both the molecules are non-polar.
(ii) Methanol and acetone : Dipole-dipole interactions as both the molecules are polar.
Question. Deffne the term, ‘molarity of a solution’. State one disadvantage in using the molarity as the unit of concentration.
Answer : Number of moles of solute dissolved in one litre solution is called molarity. It is denoted by M.
M = Number of moles of solute/Volume of solution in litre
Disadvantage in using molarity as the unit of concentration is that it depends upon temperature.
Question. The partial pressure of ethane over a saturated solution containing 6.56 × 10–2 g of ethane is 1 bar. If the solution contains 5.0 × 10–2 g of ethane, then what will be the partial pressure of the gas?
Answer : Applying the relationship,


Question. What is meant by negative deviation from Raoult’s law? Draw a diagram to illustrate the relationship between vapour pressure and mole fractions of components in a solution to represent negative deviation.
Answer : Negative deviation : For non-ideal solution, if the vapour pressure is lower, then it is said to exhibit negative deviation. A—B interactions are stronger than A—A and B—B interactions. Due to this, vapour pressure decreases which results in negative deviation. In negative deviation, intermolecular force increases, volume decreases, vapour pressure decreases and heat is released. Therefore, ΔHmix = ve, ΔVmix = – ve Example, phenol + aniline and chloroform + acetone show negative deviation.

Question. State Henry’s law correlating the pressure of a gas and its solubility in a solvent and mention two applications for the law.
Answer : Henry’s law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of liquid or solution.
Henry’s law states that, the partial pressure of the gas in vapour phase (p) is proportional to the mole fraction of the gas (x) in the solution.
p = KH⋅x where, KH = Henry’s law constant. Different gases have different KH values at the same temperature.
Applications of Henry’s law :
(i) To increase the solubility of CO2 in soda drinks and soda water, the bottle is sealed under high pressure .
(ii) To minimise the painful effects of decompression sickness in deep sea divers, oxygen diluted with less soluble helium gas is used as breathing gas.
Question. What is meant by positive deviations from Raoult’s law? Give an example. What is the sign of ΔmixH for positive deviation?
Answer : Positive deviation : For non-ideal solutions, if the vapour pressure is higher, then it is said to exhibit positive deviation. A—B interactions are weaker than A—A or B—B interactions. Due to this, vapour pressure increases which results in positive deviation. In positive deviation, intermolecular force decreases, volume increases, vapour pressures increases. enthalpy increases. Therefore, ΔHmix = +ve, ΔVmix = + ve. e.g., ethanol + acetone and carbon disulphide + acetone show positive deviation.

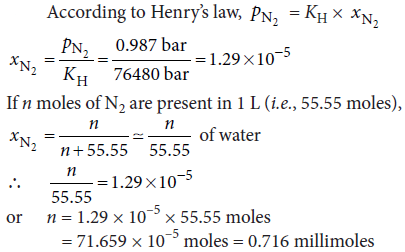
Question. If N2 gas is bubbled through water at 293 K, how many millimoles of N2 gas would dissolve in 1 litre of water? Assume that N2 exerts a partial pressure of 0.987 bar. Given that Henry’s law constant for N2 at 293 K is 76.48 k bar.
Answer :
Question. Calculate the temperature at which a solution containing 54 g of glucose, (C6H12O6 ), in 250 g of water will freeze.
(Kf for water = 1.86 K mol–1 kg)
Answer :
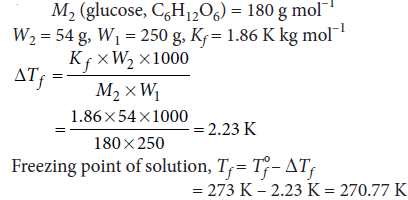
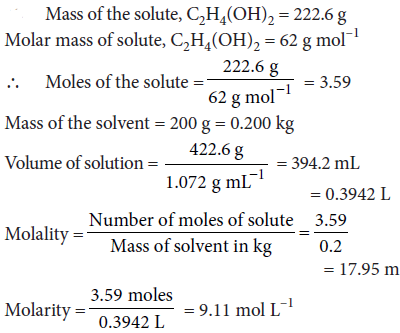
Question. An antifreeze solution is prepared from 222.6 g of ethylene glycol (C2H4(OH)2) and 200 g of water. Calculate the molality of the solution.
If the density of this solution be 1.072 g mL–1 what will be the molality of the solution?
Answer :
Question. Calculate the boiling point elevation for a solution prepared by adding 10 g of CaCl2 to 200 g of water. (Kb for water = 0.52 K kg mol–1, molar mass of CaCl2 = 111 g mol–1)
Answer : Mass of CaCl2 (W2) = 10 g
Mass of water (W1) = 200 g
Molar mass of CaCl2 (M2) = 111 g mol–1
Molal Elevation constant = 0.512 K kg mol–1
m = W2 X 1000/M2 X W1
m = 10/111 X 1000/200 = 0.450m
ΔTb = Kb m = 0.512 × 0.450 = 0.2306 K
Question. Calculate the freezing point of a solution containing 18 g glucose, C6H12O6 and 68.4 g sucrose, C12H22O11 in 200 g of water. The freezing point of pure water is 273 K and Kf for water is 1.86 K m–1.
Answer :

Question. State Raoult’s law for solutions of volatile liquids. Taking suitable examples explain the meaning of positive and negative deviations from Raoult’s law.
Answer : Raoult’s law : For a solution of volatile liquids, the partial pressure of each component in the solution is directly proportional to its mole fraction. Thus, for any component, partial vapour pressure, p ∝ x ⇒ p = p°. x
where p° = vapour pressure of pure component
x = mole fraction of that component
Positive deviation : For non-ideal solutions, if the vapour pressure is higher, then it is said to exhibit positive deviation. A—B interactions are weaker than A—A or B—B interactions. Due to this, vapour pressure increases which results in positive deviation. In positive deviation, intermolecular force decreases, volume increases, vapour pressures increases. enthalpy increases. Therefore, ΔHmix = +ve, ΔVmix = + ve. e.g., ethanol + acetone and carbon disulphide + acetone show positive deviation.
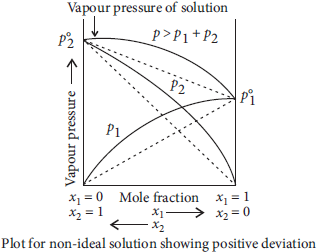
Negative deviation : For non-ideal solution, if the vapour pressure is lower, then it is said to exhibit negative deviation. A—B interactions are stronger than A—A and B—B interactions. Due to this, vapour pressure decreases which results in negative deviation. In negative deviation, intermolecular force increases, volume decreases, vapour pressure decreases and heat is released. Therefore, ΔHmix = ve, ΔVmix = – ve Example, phenol + aniline and chloroform + acetone show negative deviation.

Question. State Raoult’s law for a solution containing volatile components. Name the solution which follows Raoult’s law at all concentrations and temperatures.
Answer : Raoult’s law : For a solution of volatile liquids, the partial pressure of each component in the solution is directly proportional to its mole fraction. Thus, for any component, partial vapour pressure, p ∝ x ⇒ p = p°. x
where p° = vapour pressure of pure component
x = mole fraction of that component
Let a solution consists of two volatile liquids A and B with their mole fraction xA and xB respectively. If pA and pB are their partial vapour pressures, then,

Ideal solutions obey Raoult law at all concentrations and temperature.
Question. Calculate the amount of sodium chloride which must be added to one kilogram of water so that the freezing point of water is depressed by 3 K.
[Given : Kf = 1.86 K kg mol–1, atomic mass : Na = 23.0, Cl = 35.5]
Answer :
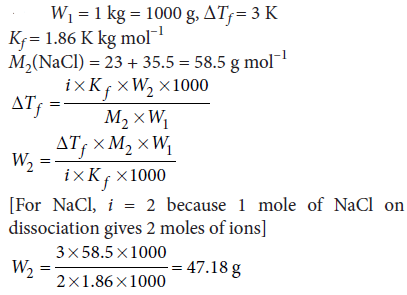
Question. Calculate the amount of KCl which must be added to 1 kg of water so that the freezing point is depressed by 2 K (the Kf for water = 1.86 K kg mol–1).
Answer :

Question. A solution of glucose (molar mass = 180 g mol–1) in water is labelled as 10% (by mass). What would be the molality and molarity of the solution?
(Density of solution = 1.2 g mL–1)
Answer : Given : Mass of solute, W2 = 10 g
Mass of solvent, W1 = 90 g
Molar mass of solute, M2 = 180 g mol–1
Density of solution = 1.2 g mL–1

