Acids Bases Salts MCQ Class 10 Science
Please refer to Chapter 2 Acids Bases Salts MCQ Class 10 Science with answers below. These multiple-choice questions have been prepared based on the latest NCERT book for Class 10 Science. Students should refer to MCQ Questions for Class 10 Science with Answers to score more marks in Grade 10 Science exams. Students should read the chapter Acids Bases Salts and then attempt the following objective questions.
MCQ Questions Class 10 Science Chapter 2 Acids Bases Salts
The Acids Bases Salts MCQ Class 10 Science provided below covers all important topics given in this chapter. These MCQs will help you to properly prepare for exams.
Question. Egg shell is made up of
(a) CaCO3
(b) CaO
(c) Ca(OH) 2
(d) CaCl 2
Answer
A
Question. Rain is called acid rain when its:
(a) pH falls below 7
(b) pH falls below 6
(c) pH falls below 5.6
(d) pH is above 7
Answer
C
Question. Which of the following is not a acidic salt?
(a) CuSO4
(b) NH 4 Cl
(c) FeCl 3
(d) CH 3 COONa
Answer
D
Question. Which one of the following types of medicines is used for treating indigestion?
(a) Antibiotics
(b) Analgesic
(c) Antacid
(d) Antiseptic
Answer
C
Question. Sodium hydroxide turns phenolphthalein solution
(a) pink
(b) yellow
(c) colourless
(d) orange
Answer
A
Question. Sodium hydroxide is a
(a) weak base
(b) weak acid
(c) strong base
(d) strong acid
Answer
C
Question. Tooth enamel is made up of
(a) calcium phosphate
(b) calcium carbonate
(c) calcium oxide
(d) potassium
Answer
A
Question. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking power
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer
D
Question. Assertion: Universal indicator gives green colour with distilled water.
Reason: pH of distilled water is 7 and it is neutral and universal indicator gives green colour with neutral solution.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
Answer
A
Question. A drop of liquid sample was put on the pH paper, paper turned blue. The liquid sample must be of
(a) Lemon Juice
(b) HCl
(c) Sodium bicarbonate
(d) Ethanoic acid.
Answer
C
Question. Calcium phosphate is present in tooth enamel. Its nature is
(a) basic
(b) acidic
(c) neutral
(d) amphoteric
Answer
A
Question. Setting of Plaster of Paris takes place due to
(a) Oxidation
(b) Reduction
(c) Dehydration
(d) Hydration
Answer
D
Question. You have four test tubes, A, B, C and D containing sodium carbonate, sodium chloride, lime water and blue litmus solutions respectively. Out of these the material of which test tube / test tubes would be suitable for the correct test of acetic acid / ethanoic acid.
(a) Only A
(b) A and B
(c) B and C
(d) A and D.
Answer
D
Question. In general, salts
(a) Are ionic compounds
(b) Contain hydrogen ions
(c) None of these
Answer
A
Question. A substance that will not give carbon-dioxide on treatment with dilute acid is:
(a) Marble
(b) Lime stone
(c) Baking soda
(d) Limewater
Answer
D
Question. A student took two test tubes containing 2ml of dilute hydrochloric acid and added zinc granules to test tube (A) and solid sodium carbonate to test tube (B) as shown below The correct observation would be:
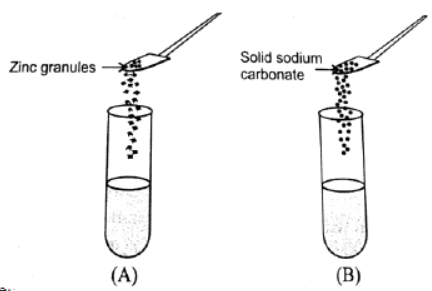
(a) Rapid reaction in both test tubes
(b) Slow reaction in (A) and rapid reaction in (B)
(c) Rapid reaction in (A) but a slow reaction in (B)
(d) No reaction in any of the test tubes
Answer
A
Question. When aqueous solutions of an acid and a base are mixed
(a) No reaction occurs
(b) A new acid and a new base are formed
(c) A salt and water are formed
(d) An acid and a salt are formed
Answer
C
Question. A solution turns red litmus blue; the pH is likely to be:
(a) 1
(b) 4
(c) 5
(d) 14
Answer
D
Question. A 10 ml of solutions of NaOH is found to be completely neutralised by 8 ml. of a given solution of HCl. If we take 20 ml. of the same solution of NaOH, the amount of HCl solution (the same solution as before) required to neutralize it will be:
(a) 4 ml
(b) 8 ml
(c) 12 ml
(d) 16 ml
Answer
D
Question. Sodium carbonate is a basic salt because it is a salt of:
(a) Strong acid and strong base
(b) Weak acid and weak base
(c) Strong acid and weak base
(d) Weak acid and strong base.
Answer
D
Question. Common salt besides being used in kitchen can also be used as the raw material for making:
1) Hydrogen
2) Nitrogen
3) Chlorine
4) Slaked lime
(a) (1) and (2)
(b) (1), (2) and (4)
(c) (1) and (3)
(d) (1), (3) and (4)
Answer
C
Question. Na 2 CO 3 . 10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
Answer
A
Question. Sodium carbonate is a basic salt because it is a salt of a
(a) strong acid and strong base
(b) weak acid and weak base
(c) strong acid and weak base
(d) weak acid and strong base
Answer
D
Question. Lime water reacts with chlorine to give
(a) bleaching powder
(b) baking powder
(c) baking soda
(d) washing soda
Answer
C
Question. In the following reaction, identify the salt formed
NH4OH (aq) + H2SO4 (aq) → _____ + 2H2O (l)
(a) NH4NO3
(b) (NH4)2SO4
(c) (NH4)3PO4
(d) (NH4)2S
Answer
B
Question. The figure given below represents the experiment carried out between con(c) sulphuric acid and sodium chloride, which react with each other to form HCl gas.

Blue litmus paper is brought near the mouth of the delivery tube to check the presence of HCl acid but no change is observed in the color of litmus paper because:
(a) The litmus paper used is dry
(b) The litmus paper used is moist
(c) Blue litmus paper does not change its color with an acid
(d) The litmus paper is kept very close to the mouth of the delivery tube
Answer
A
Question. Some fruits like mango, lemon, raw grapes, orange, et(c), have a sour taste due to the presence of:
(a) Acetic acid
(b) Citric acid
(c) Lactic acid
(d) Oxalic acid
Answer
B
Question. Which of the following phenomena occur, when a small amount of acid is added to water?
i. Ionisation
ii. Neutralisation
iii. Dilution
iv. Salt formation
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
B
Question. Zinc granules on treating with an acid X, form the zinc sulphate (ZnSO4) salt along with the evolution of a gas Y which burns with a pop sound when brought near to a burning candle. Identify the acid X and gas evolved Y.
(a) X- Sulphuric acid and Y- Oxygen gas
(b) X- Hydrochloric acid and Y- Oxygen gas
(c) X- Sulphuric acid and Y- Hydrogen gas
(d) X- Hydrochloric acid and Y- Hydrogen gas
Answer
C
Question. Copper sulphate crystals when heated strongly, lose their water of crystallization to give anhydrous copper sulphate accompanied by a change in color from:
(a) Blue to green
(b) Blue to white
(c) Blue to sky blue
(d) Blue to grey
Answer
B
Question. Which of the following indicators turn red in an acidic solution?
i. Phenolphthalein
ii. Litmus
iii. Turmeric
iv. Methyl orange
Choose the correct option:
(a) (i) and (ii)
(b) (ii) and (iii)
(c) Only (ii)
(d) (ii) and (iv)
Answer
D
Question. Dilute acid does not produce carbon dioxide on being treated with:
(a) Marble
(b) Lime
(c) Baking soda
(d) Limestone
Answer
B
Question. Which among the following represents the chemical formula for ‘Plaster of Paris’?
(a) CaSO4. 2H2O
(b) CaSO4. 1/2H2O
(c) CaSO4. H2O
(d) CaSO4. 10H2O
Answer
B
Question. The sample of soil from a particular place was tested for its pH value. It came out to be 5. Which one of the following should be added to the soil to make it suitable for the plant growth?
i. Calcium chloride
ii. Calcium Hydroxide
iii. Calcium oxide
Choose the correct option:
(a) Both (i) and (ii)
(b) Both (ii) and (iii)
(c) Only (i)
(d) Only (iii)
Answer
B
Question. Which of the following salt will give acidic solution when dissolved in water?
(a) NH4Cl
(b) NaCl
(c) Na2CO3
(d) CH3COONa
Answer
A
Question. Identify the products of the following reaction:
CaCO3 + 2HCI → ……….. + ……..
(a) Calcium hydrogencarbonate and chlorine gas
(b) Calcium chloride and water
(c) Calcium oxide, carbon dioxide and water
(d) Calcium chloride, carbon dioxide and water
Answer
D
Question. Which one of the following salts will dissolve in water to form an alkaline solution?
(a) Potassium carbonate
(b) Sodium chloride
(c) Sodium carbonate
(d) Potassium sulphate
Answer
A
Question. An ant’s sting can be treated with …………which will neutralise the effect of the chemical injected by the ant’s sting into our skin. Choose the correct option from the following to be filled in the blank space:
(a) Methanoic acid
(b) formic acid
(c) Baking soda
(d) Caustic soda
Answer
C
Question. Bleaching powder is used as a disinfectant for water to:
(a) Make water tastier
(b) Remove all the dirt from water
(c) Make water germ-free
(d) Make water clear
Answer
C
Question. NaHCO, formed by reaction of
(a) NaOH + H 2 CO3
(b) NaCl + H 2 CO3
(c) Na 2 CO 3 + HCl
(d) NaOH + Na 2 CO3
Answer
A
Question. Chemical formula of baking soda is:
(a) MgSO4
(b) Na2CO3
(c) NaHCO 3
(d) MgCO 3
Answer
C
Question. At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
Answer
B
Question. What is the pH range of human body?
(a) 7.0 – 7.8
(b) 7.2 – 8.0
(c) 7.0 – 8.4
(d) 7.2 – 8.4
Answer
A
Question. Tomato is a natural source of which acid?
(a) Acetic acid
(b) Citric acid
(c) Tartaric acid
(d) Oxalic acid
Answer
D
Question. Assertion: Bleaching power liberate chlorine when kept in atmosphere.
Reason: CaOCl2 reacts with CO2 present in atmosphere to form CaCO 3 and chlorine gas.
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
(e) Both A and R are false.
Answer
A
Question. Brine is an
(a) aqueous solution of sodium hydroxide
(b) aqueous solution of sodium carbonate
(c) aqueous solution of sodium chloride
(d) aqueous solution of sodium bicarbonate
Answer
C
Question. In terms of acidic strength, which one of the following is in the correct increasing order?
(a) Water < Acetic acid < Hydrochloric acid
(b) Water < Hydrochloric acid < Acetic acid
(c) Acetic acid < Water < Hydrochloric acid
(d) Hydrochloric acid < Water < Acetic acid
Answer
A
Question. Phenolphthalein exhibits which colour with a base?
(a) Remains colourless
(b) Pink
(c) Red
(d) Green
Answer
B
Question. NaOH is obtained by electrolysis of
(a) Aq. solution of NaCl
(b) Aq. Na2CO3
(c) Aq. NaHCO3
(d) Molten NaCl
Answer
A
Question. The chemical name of bleaching powder is
(a) calcium hypo oxychloride
(b) calcium oxychloride
(c) calcium chloride
(d) calcium chloro oxide
Answer
B
Question. Phenolphthalein exhibits which colour with an acid?
(a) Remains colourless
(b) Pink
(c) Red
(d) Green
Answer
A
Question. Methyl orange which is an indicator turns into which colour with an acid?
(a) Red
(b) Yellow
(c) Pink
(d) No colour
Answer
A
Question. Which statement is correct?
(a) Organic acids are obtained from natural sources.
(b) Inorganic acids are prepared in laboratory.
(c) Bee sting contains formic acid.
(d) All of the above.
Answer
D
Question. What happens when acid is mixed with water?
(a) Heat is evolved
(b) Heat is absorbed
(c) Concentration of acid increases
(d) All of the above
Answer
A
Question. Methyl orange which is an indicator turns into which colour with a base?
(a) Red
(b) Yellow
(c) Pink
(d) No colour
Answer
B
Question. What the original colour of methyl orange solution which is an indicator?
(a) Yellow
(b) Orange
(c) Pink
(d) Red
Answer
B
Question. Which of the following compound is formed when zinc reacts with hydrochloric acid?
(a) Zinc sulphate
(b) Zinc chloride
(c) Zinc carbonate
(d) Zinc hydroxide
Answer
B
Question. What happens when excess of carbon dioxide gas is passed through lime water?
(a) Lime water first turns milky and then colourless
(b) Lime water turns bluish
(c) Lime water turns milky
(d) Lime water turns blackish
Answer
A
Question. What is the nature of non-metallic oxides?
(a) Basic
(b) Acidic
(c) Neutral
(d) None of the above
Answer
B
Question. A basic solution is added to a test tube. A blue and red litmus paper is dipped into the basic solution. What will happen to both litmus papers?
(a) Blue litmus paper: changes colour; red litmus paper: no colour change
(b) Blue litmus paper: changes colour; red litmus paper: changes colour
(c) Blue litmus paper: no colour change; red litmus paper: changes colour
(d) Blue litmus paper: no colour change; red litmus paper: no colour change
Answer
C
Question. A solution of pH2 is filled in two separate beakers. A few drops of methyl orange and phenolphthalein are added into separate solutions. How will the colour of the indicators change?
(a) Methyl orange: red; phenolphthalein: pink
(b) Methyl orange: orange; phenolphthalein: colourless
(c) Methyl orange: red; phenolphthalein: colourless
(d) Methyl orange: orange; phenolphthalein: pink
Answer
C
Question. Bases on ionisation release
(a) Hydrogen ions
(b) Sodium ions
(c) Chlorine ions
(d) Hydroxide ions
Answer
D
Question. In the following schematic diagram for the preparation of hydrogen gas as shown in below Figure, what would happen if following changes are made?

(a) In place of zinc granules, same amount of zinc dust is taken in the test tube.
Answer
Fast
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken.
Answer
Hydrogen
(c) In place of zinc, copper turnings are taken.
Answer
No Displacement
(d) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated
Answer
Hydrogen
Question. When magnesium and hydrochloric acid react, they produce
(a) Oxygen and magnesium chloride
(b) Chlorine and magnesium oxide
(c) Hydrogen and magnesium chloride
(d) Hydrogen and magnesium
Answer
C
Question. Sodium Carbonate when added to acetic acid evolves a gas. Which of the following are true about the gas evolved?
(i) It turns lime water milky
(ii) It puts out burning splinter
(iii) It dissolves in sodium hydroxide solution
(iv) It has pungent smell.
(a) 1 & 2
(b) 1,2 & 3
(c) 2, 3 & 4
(d) 1 & 4.
Answer
B
Question. A solution of a weak acid when mixed with a solution of a strong base forms:
(a) Acidic solution
(b) Basic solution
(c) Salt and Water
(d) Neutral solution.
Answer
B
Question. An element common to all acids is
(a) Chlorine
(b) Nitrogen
(c) Oxygen
(d) hydrogen
Answer
D
Question. An aqueous solution turns blue litmus solution red. Excess addition of which solution of the following solution would reverse the change:
(a) Acetic acid
(b) Limewater
(c) Ammonium Hydroxide solution
(d) Hydrochloric Acid.
Answer
C
Question. Identify the compound X on the basis of the reactions given below. Also, write the name and chemical formulae of A, B and C.

Answer
X. NAOH
A. Sodium Zincate
B. Sodium
Chloride
C. Sodium Acetate
Question. Four students were asked by their teacher to arrange the set up I-IV as given below and identify the gas evolved in each case, if any.

After observation, they arrived at the following inferences and recorded their observations in the form of a table as given below:

Find which student recorded the correct observation.
Answer
Student C
Question. Calcium phosphate is present in tooth enamel. Its nature is
(a) basic
(b) acidic
(c) neutral
(d) amphoteric
Answer
A
Question. What happens when hydrogen carbonate reacts with an acid?
(a) Carbon monoxide
(b) Carbonic acid gas
(c) Carbon dioxide gas
(d) Hydrochloric acid gas
Answer
C
Question. What happens when carbon dioxide gas is passed through lime water?
(a) Lime water turns milky
(b) Lime water turns colourless
(c) Lime water turns bluish
(d) Lime water turns black
Answer
A
Question. Which one of the following can be used as an acid-base indicator by a visually impaired (blind) student?
(a) Litmus
(b) Turmeric
(c) Vanilla essence
(d) Petunia leaves
Answer
C
Question. Which of the following compound is formed when zinc reacts with sodium hydroxide?
(a) Zinc hydroxide
(b) Sodium zincate
(c) Zinc hydrogenate
(d) No reaction takes place
Answer
B
Question. Which of the following gas is formed when an acid reacts with metal carbonate?
(a) Carbon monoxide
(b) Carbonic acid gas
(c) Carbon dioxide gas
(d) Hydrochloric acid gas
Answer
C
Question. A visually challenged student, has to perform a lab test to detect the presence of acid in a given solution. The acid-base indicator preferred by him will be:
(a) Blue litmus
(b) Clove oil
(c) Red cabbage extract
(d) Hibiscus extract
Answer
B
Question. Calcium phosphate is present in tooth enamel. Its nature is:
(a) Basic
(b) Acidic
(c) Neutral
(d) Amphoteric
Answer
A
Question. Baking soda is a mixture of:
(a) Sodium carbonate and acetic acid
(b) Sodium carbonate and tararic acid
(c) Sodium hydrogen carbonate and tartaric acid
(d) Sodium hydrogen carbonate and acetic acid
Answer
C
Question. Which of the following gives the correct increasing order of acid strength?
(a) Water < acetic acid < hydrochloric acid
(b) Water < hydrochloric acid < acetic acid
(c) Acetic acid < water < hydrochloric acid
(d) Hydrochloric acid < water < acetic acid
Answer
A
Question. The chemical formula for plaster of Paris is :1
(a) CaSO4.2H2O
(b) CaSO4.H2O
(c) CaSO4. 1/2 H2O
(d) 2CaSO4.H2O
Answer
C
Question. Sodium hydrogen carbonate when added to acetic acid evolves a gas. Which of the following statements are true about the gas evolved?
(i) It turns lime water milky.
(ii) It extinguishes a burning splinter.
(iii) It dissolves in a solution of sodium hydroxide.
(iv) It has a pungent odour.
(a) (i) and (ii)
(b) (i), (ii) and (iii)
(c) (ii), (iii) and (iv)
(d) (i) and (iv)
Ans. (b) (i), (ii) and (iii)
Answer
B
Question. What happens when the solution of an acid is mixed with the solution of a base in a test tube?
(i) The temperature of the solution increases
(ii) The temperature of the solution decreases
(iii) The temperature of the solution remains the same
(iv) Salt formation takes place
(a) Only (i)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer
D
Question. Identify the basic salt from the following salts:
(a) Na2CO3
(b) NH4Cl
(c) NaNO3
(d) KCl
Answer
A
Question. Which of the following is/are true when HCl(g) is passed through water?
(i) It does not ionise in the solution as it is a covalent compound.
(ii) It ionises in the solution.
(iii) It gives both hydrogen and hydroxyl ions in the solution.
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule.
(a) Only (i)
(b) Only (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
C
Question. Which of the following solutions in water does not conduct electricity?
(a) Hydrochloric acid
(b) Sodium chloride
(c) Glucose
(d) Sulphuric acid
Answer
C
Question. Which of the following will turn phenolphthalein pink?
(a) NaOH(aq)
(b) HCl(aq)
(c) CH3COOH(aq)
(d) H2O
Answer
A
Question. Which among the following is not a base?
(a) NaOH
(b) KOH
(c) NH4OH
(d) C2H5OH
Answer
D
Question. Common salt besides being used in kitchen can also be used as the raw material for making:
(i) Washing soda (ii) Bleaching powder
(iii) Baking soda (iv) Slaked lime
(a) (i) and (ii)
(b) (i), (ii) and (iv)
(c) (i) and (iii)
(d) (i), (iii) and (iv)
Answer
C
Question. Sodium carbonate is a basic salt because it is a salt of:
(a) Strong acid and strong base
(b) Weak acid and weak base
(c) Strong acid and weak base
(d) Weak acid and strong base
Answer
D
Question. Calcium phosphate is present in tooth enamel.
Its nature is:
(a) Basic
(b) Acidic
(c) Neutral
(d) Amphoteric
Answer
A
Question. Match the chemical substances given in Column I with their appropriate application given in Column II:
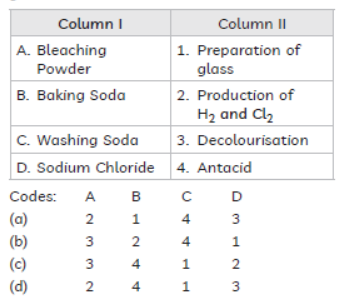
Answer
C
Question. Which of the following statements is correct about an aqueous solution of an acid and a base?
(i) The higher the pH, the stronger the acid
(ii) The higher the pH, the weaker the acid
(iii) The lower the pH, the stronger the base
(iv) The lower the pH, the weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
D
Question. Which of the following substances will not give carbon dioxide on treatment with dilute acid?
(a) Marble
(b) Limestone
(c) Baking soda
(d) Lime
Answer
D
Question. Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained? (You may use colour guide in the figure given below).
(a) Red
(b) Yellow
(c) Yellowish green
(d) Blue
OR
Which of the following is/are true when HCl(g) is passed through water:
(i) It does not ionise in the solution as it is a covalent compound
(ii) It ionises in the solution
(iii) It gives both hydrogen and hydroxyl ion in the solution
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
(a) Only (i)
(b) Only (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
C
Question. Which of the following phenomena occur, when a small amount of acid is added to water?
(i) Ionisation (ii) Neutralisation
(iii) Dilution (iv) Salt formation
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
B

We hope you liked the above Acids Bases Salts MCQ Class 10 Science. In case you have any questions please put them in the comments box below and our teachers will provide you a response.


