Carbon and Its Compound Notes for Class 10 Science
Read these Carbon and Its Compounds Class 10 Notes pdf. These Notes of carbon and its compounds class 10 are prepared by our expert teachers on the latest exam pattern of the CBSE Board Exam. It will help you to make your preparation better to score good marks in your exam.
Class 10 Carbon and Its compounds Notes are great for preparing for the CBSE Class 10 board examinations. CBSE Class 10 Science Syllabus is much bigger and requires concentrated efforts on the part of the student to face the examinations and pop out a success.
Carbon and Its Compounds Class 10 Notes Pdf consist of all of the concept and important topics which help you in revision. Each explanation is provided with all the assumptions and good judgment used to determine the conclusion. This will allow the students to study and understand each concept even though they are preparing for the first time.
Important Terms & Concepts
- Carbon: Its atomic number is 6. Its mass number is 12.0. Its atomic mass is 12.011. Its melting point is 3550℃ and boiling point is 4830℃. It occurs in Free State as well as in combined state. 70% of our body is made up of carbon. It forms largest number of compounds. The earth’s crust contains only 0.02% of carbon. It can Lose 4 electron easily cant
gain four electrons easily but can share four electrons to form covalent bond so as to become stable.
Organic Compounds: Those compounds which consist of carbon essentially and hydrogen mostly along with other elements like oxygen, sulphur, nitrogen, halogens, etc. are called organic compounds.
Chemical Bond: it is a force of attraction which holds the two atoms together.
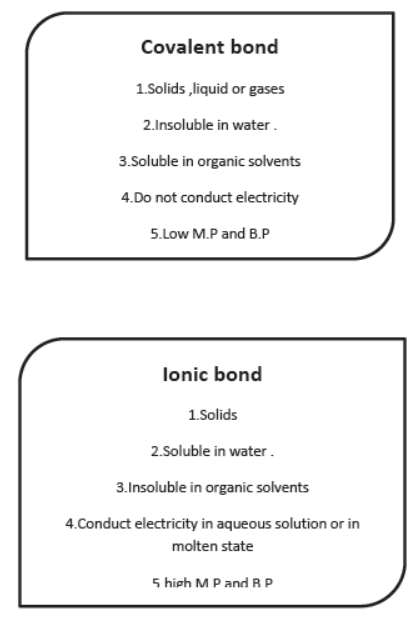
Covalent Bond: It is the bond formed by equal sharing of electrons, e.g., Hydrogen has one valence electron. It can share one valence electron with other hydrogen atom to form H2 molecule so as to acquire nearest noble gas configuration. The bond between two hydrogen atoms formed by sharing one electron each is called covalent bond.
Self-Combination :The property of self-combination of carbon atoms to form long chains is useful to us because it gives rises to an extremely large number of carbon compounds.
Note: the formation of strong bonds by carbon atoms among themselves and with other elements makes the carbon compounds exceptionally stable.
Covalency of Carbon: Carbon has four valence electrons. It cannot lose four electrons since very high amount of energy will be required to lose four electrons to form ion. There is strong force of attraction between nucleus and valence electrons.
Carbon cannot gain four electrons to form ion because six protons cannot hold ten electrons easily and there will be strong interelectronic repulsion.
Carbon can share four electrons easily with other atoms of carbon and other elements to acquire stable electronic configuration.
Occurrence Of carbon :
• In free state e.g., Diamond and graphite
• In the combined state e.g ., Coal, petroleum ,carbonates.
Single Covalent Bond: It is a bond formed by sharing one electron by each of the atoms. It is represented by a line between two atoms.
Hydrogen Molecule (H2): When two atoms of hydrogen share one electron each a single covalent bond is formed as shown below.

(Single covalent bond between two hydrogen atoms)
Chlorine Molecule (Cl2): Chlorine has seven valence electrons. It can share one electron with other chlorine atom to form chlorine molecule.

(Single covalent bond between two hydrogen atoms)
Hydrogen Fluoride (HF): When one hydrogen atom shares one electron with one electron of fluorine, hydrogen acquires two electrons whereas fluorine acquires eight electrons and becomes stable. They form single covalent bond.

(Single covalent bond between two hydrogen atoms)
Water (H2O): In formation of water, each hydrogen atom shares one electron with oxygen atom so that oxygen completes its octet and hydrogen acquires nearest noble gas configuration.

Ammonia (NH3): Nitrogen has live valence has electrons. It shares one electron with each of the three hydrogen atoms to form ammonia.

Methane (CH4): Carbon has four valence electrons. It needs four electrons to complete its octet. It shares four electrons with four hydrogen atoms and forms four single covalent bonds.
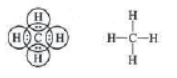
Ethane (C2H6): In ethane, two carbon atoms share one electron each forming single covalent bond with each other.
Each carbon atom shares one electron with three hydrogen atoms to complete their octet, e.g.,
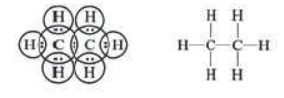
Methyl chloride (CH3Cl): Carbon has four valence electrons. It shares one electron with chlorine atom and one electron with each of three hydrogen atoms forming four single bonds. Its IUPAC name is chloromethane.

Carbon tetrachloride (CCl4): Carbon shares one electron with each of four chlorine atoms forming four single covalent bonds. Its IUPAC name is tetrachloromethane.

Double Covalent Bond: When two atoms share two electrons each to acquire stable electronic configuration, double covalent bond is formed. It is denoted by = (two lines).
Oxygen Molecule (O2): When two oxygen atoms share two electrons each to complete their octet, double covalent bond is formed.

(A double covalent bond between two oxygen atoms)
Ethene (C2H4): When two carbon atoms share two electrons with each other and each carbon shares two electrons with two hydrogen atoms, they complete their octet and form double covalent bond between two carbon atoms. Its common name is ethylene.

Carbon dioxide (CO2): Carbon has four valence electrons. It shares two electrons with one of the oxygen and two electrons with other atom of oxygen to form double covalent bond.

Triple Covalent Bond: When an atom shares three valence electrons with each other or other atom, triple covalent bond is formed. It is denoted by ≡ (three lines).
Nitrogen (N2): Nitrogen has five valence electrons. It needs three more electrons to complete its octet. It shares three electrons with other atom of nitrogen to form triple covalent bond.
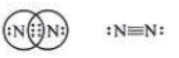
(Triple covalent bond between two nitrogen atoms)
Ethyne (C2H2): When two carbon atoms share three electrons with each other and each carbon shares one electron with hydrogen atom, they complete their octet and form triple covalent bond with each other. Its common name is acetylene.

Properties of Covalent Compounds:
I) Physical State: Covalent compounds can exist in solid, liquid as well as gaseous state e.g., CH4 is gas, CHCl3 (chloroform) is liquid, CHO6 (glucose) is solid.
II) Solubility:
a) They are generally insoluble in water and in polar solvents because they cannot form ions in aqueous solution.
b) They are soluble in non-polar organic solvents like ether, benzene, CCl4 (carbon tetrachloride), CS2 (carbon disulphide), CHCl3 (chloroform), acetone, etc.
III) Electrical Conductivity: Covalent compounds are poor conductors of electricity because they do not contain ions or free electrons for conduction of electricity, e.g., CCl4. C6H6 (benzene), C6H5CH3 (toluene) do not conduct electricity.
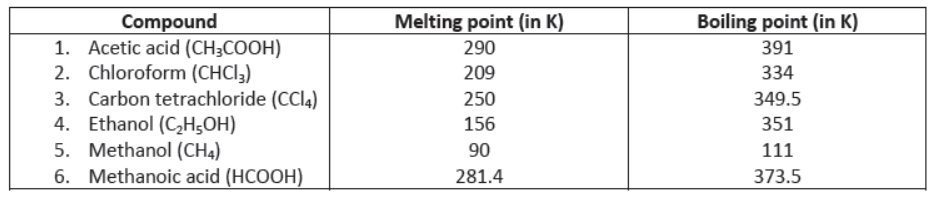
IV) Melting and Boiling Points: Melting and boiling points of covalent compounds are low due to weak forces of attraction between molecules. Less energy is required to overcome these forces of attraction. e.g.,
Isotopes of Carbon: Naturally occurring carbon has two stable isotopes 12/6C(98.9%) and 13/6(1.1%) in addition to traces of radioactive 14/6C isotope which is used to determine the age of archaeological specimen of organic origin. The isotope 12/6C is the international standard for atomic mass measurement and assigned a mass of 12.00000 units.
Allotropy: It is a property due to which an element can exist in more than one form which differ in physical properties but have similar chemical properties, e.g., carbon, sulphur, phosphorus, oxygen show allotropy.
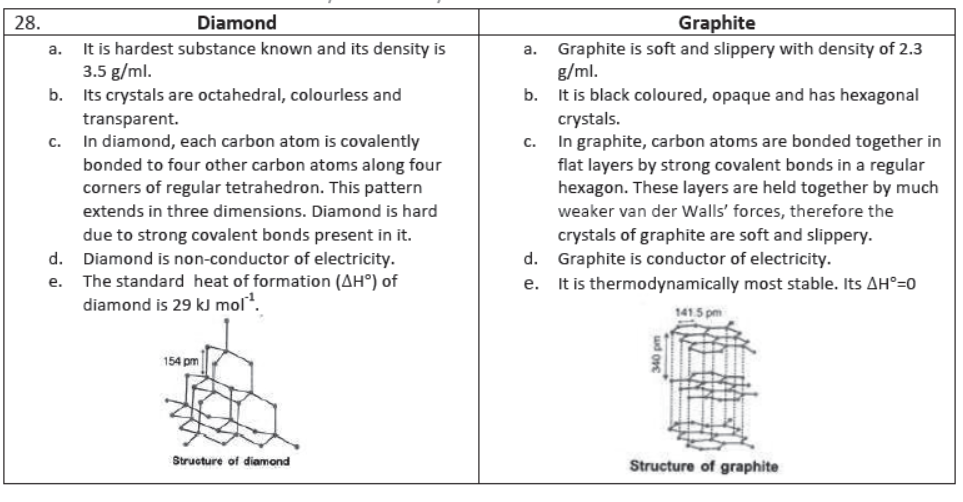
Allotropes of Carbon: The carbon exists both in crystalline and amorphous forms. The two well-known allotropes of carbon are diamond and graphite.
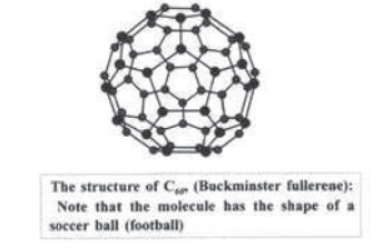
Fullerenes: A third form of carbon known as fullerenes were discovered by H. W. Kroto, R. F. Curt and R. E. Smalley. Fullerenes consist of hollow cage of carbon atoms. They are large spheroidal molecules composition C2n two important members of this family are C60 and C70 . The 1996 Nobel Prize was awarded to above scientists for the discovery of fullerenes.
Note: Buckminsterfullerene is a dark ,solid at room temperature .Diamond is extremely hard and Graphite is extremely soft whereas fullerence is neither very hard nor very soft.
28.
Unique Nature of Carbon: Carbon has small size and therefore can from strong covalent bonds with other atoms. It forms maximum number of compounds. Our body is made up of carbon compounds like proteins, fats, nucleic acids.
Catenation: It is a property due to which carbon can form bonds with other atoms o carbon. Carbon shows the property of catenation to maximum extent because it is small in size and can form strong covalent bonds.
Tetravalency of Carbon: Carbon has four valence electrons. It can share four electrons with other atoms of carbon as well as oxygen, hydrogen, nitrogen, sulphur and halogens.
Large Number of Organic Compounds: They are due to tetravalency of carbon and property of catenation.
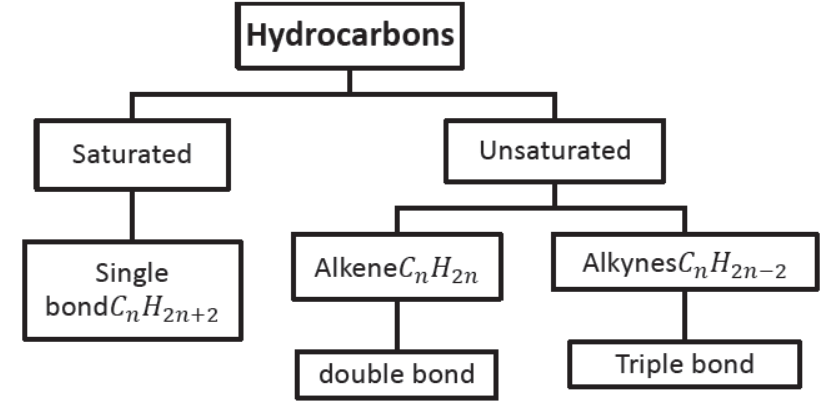
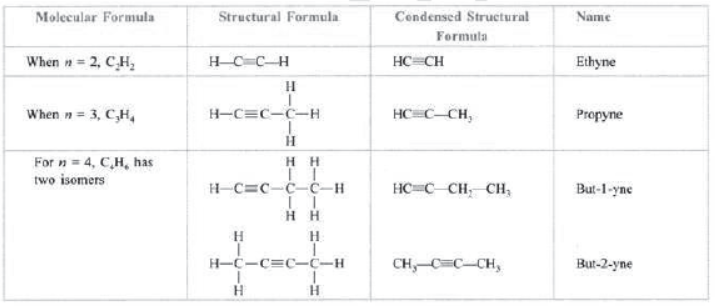
Hydrocarbons: Those compounds which contain carbon and hydrogen only are called hydrocarbons, e.g., CH4 (methane), C2H6 (ethane). C2H4 (ethene), C2H2 (ethyne), etc.
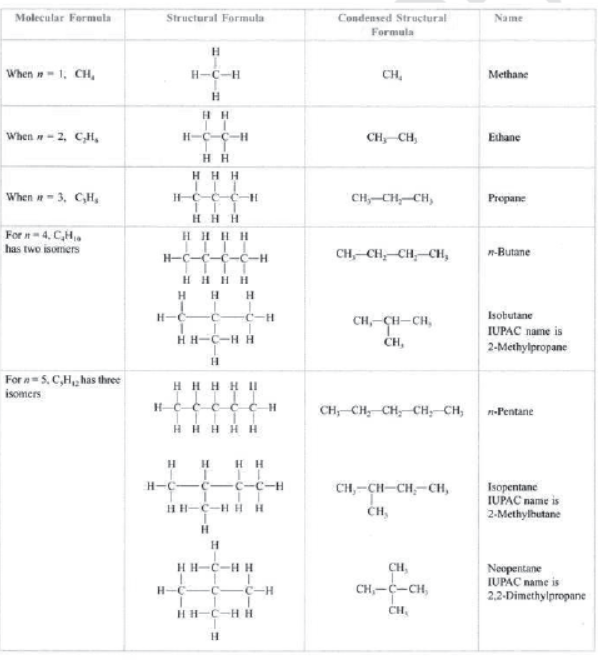
Saturated hydrocarbons: Those hydrocarbons which contain single bonds only are called saturated hydrocarbons, e.g., CH4 (methane), C2H6 (ethane), C3H8 (propane), C4H10 (butane), etc.
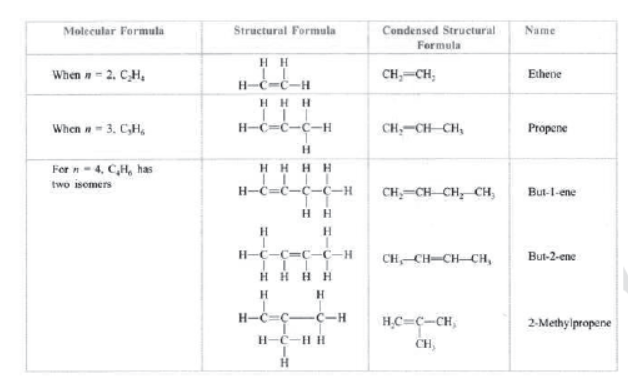
Unsaturated hydrocarbons: Those hydrocarbons in which valency of carbon is satisfied by double or triple bond are called unsaturated hydrocarbons, e.g., C2H4 (ethene), C3H6 (propene), C2H2 (ethyne), etc.
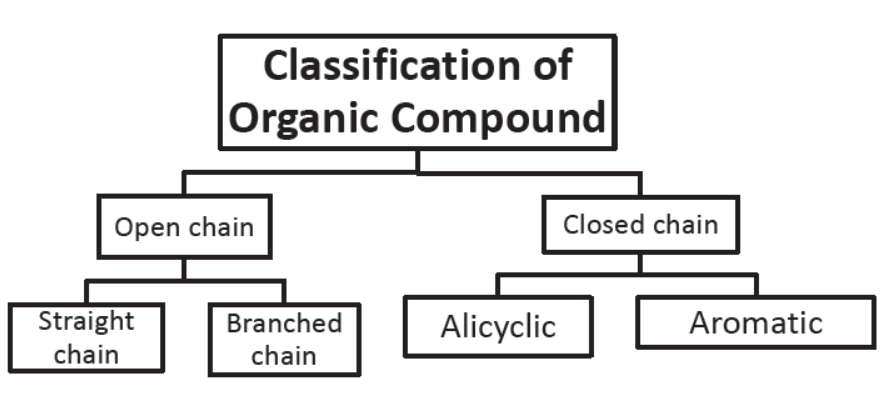
Straight Chain Compounds: Those compounds which contain straight carbon chains are called straight chain compounds.
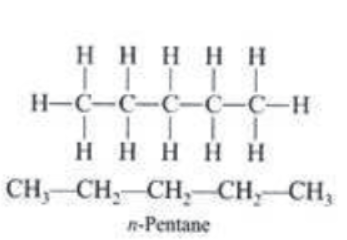
Branched Chain Compounds: Those compounds which are branched are called branched chain compounds, e.g.,
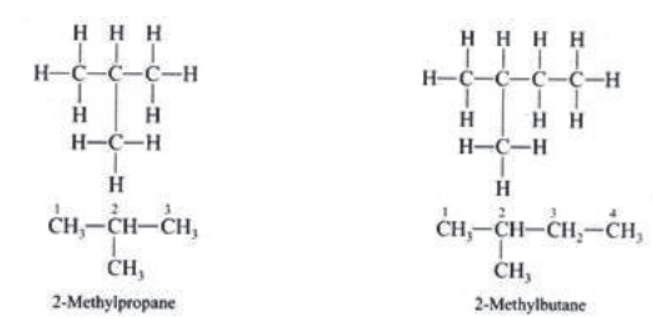
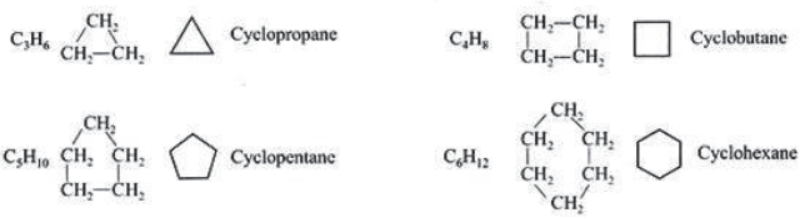
Closed Chain Compounds or Ring Compounds: Cycle compounds are called closed chain or ring compounds, e.g.,
Aromatic Compounds: Benzene and its derivatives (which contain benzene ring) are called aromatic compounds, e.g., C6H6
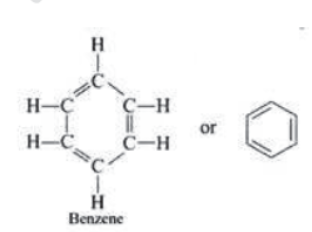
Alkanes: All compounds in which carbon and hydrogen are attached with single bonds are called alkanes.
The general formula of alkane from which all the members of family can be derived is CnH2n+2n , e.g.,
CH4.C2H6, C3H8.C4H10, C5H12, C6H14, etc.
The saturated hydrocarbon is not very reactive hence called as paraffins.
Alkenes: Those unsaturated hydrocarbons which have one or more double bonds are called alkenes. Their general formula is CnH2n , e.g., C2H4 (ethene), C3H6 (propene), C4H8 (butane), C5H10 (pentene), C6H10 (hexane), etc.
Alkynes: Those unsaturated hydrocarbons which contain one or more triple bonds are called alkynes. The general formula of alkynes is CnH2n– , e.g., C2H2 (ethyne), C3H4 (propyne), C4H6 (butyne), C5H8 (pentyne), C6H10 (hexyne), etc.
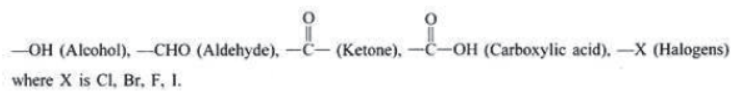
Functional Group: It is atom or group of atoms or reactive part of compound which largely determines the chemical properties of compound, e.g.,
Homologous Series: It is a series of compounds which are derived from same general formula, having same functional group, similar chemical properties and show gradation in physical properties. Each member differs from successive member by —CH2—. The difference in molecular weight between two successive members is 14 u.
Characteristics of Homologous Series:
• Same general formula
• Similar methods of preparation
• Same functional group
• Similar chemical properties
• Gradation in physical properties .
• Gradation in solubility.
• Successive members differ by 𝐶𝐻2
• Successive members differ by 14µ
IUPAC stands for International Union of Pure and Applied Chemistry:
IUPAC names are used for International communication. Rules for IUPAC Naming of Organic Compounds.
I) Select the possible longest chain containing the functional group.

II) The number of carbon atoms in the parent compounds is denoted by proper prefix:
meth for one eth for two prop for three but for four pent for five hex for six hept for seven oct of eight non for nine dec for ten e.g., in CH3—CH2—CH2— CH2—CH2—CH3 the parent chain contains six carbon atoms, it is called hexane. ane is the suffix for alkanes (saturated hydrocarbons) having single bonds only.
III) Groups attached to the parent chain are indicated by their names and prefixing the number of carbon to which they are attached in parent chain.

IV) The counting of carbon chain is done in such a way that the carbon attached to the alkyl group or functional group gets the minimum number, e.g.,

V) If more than one identical group is attached to same or different carbon atoms, prefix the numbers of carbon to which they are attached. The number of these groups are indicated as: di for two, tri for three, tetra for four and so on e.g.,

VI) For double bond in alkenes suffix-ene, for triple bond suffix-yne is used in alkynes. In alkenes and alkynes, number of carbon atoms after which double or triple bond is present in also prefixed, e.g.,

Alkanes: They have general formula CnH2n+2 where n is the number of carbon atoms. —ane is the suffix used in alkanes.
Alkenes: They have general formula CnH2n where n is the number of carbon atoms. —ene is the suffix used in alkenes.
Alkynes: They have general formula CnH2n-2 where n is the number of carbon atoms. —yne is the suffix used in alkynes.
Alcohols: Alcohols are carbon compounds containing –OH group attached to carbon atom. The general formula of alcohol is R−OH where ‘R’ is an alkyl group and –OH is a functional group.
The name of alcohol is derived by replacing –e in the name of alkane from which it is derived by the suffix – ol. For example, methanol (CH3OH), an alcohol is derived by substituting ‘H’ of methane by – OH.

Alkyl halides: They have general formula CnH2n-1 X, where X is Cl, Br, I, F.
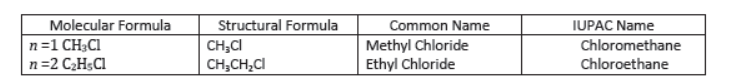
Aldehydes and Ketones: Aldehydes and ketones are compounds containing carbonyl () group. In aldehydes, carbon of
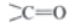
group is attached to an alkyl group and a hydrogen atom. In ketones, carbon of carbonyl group is attached to two alkyl groups. The two alkyl groups may be same or different. For example:
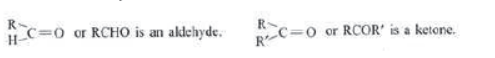
Where R and R’ are different alkyl groups. They can be same also.
Aldehydes are named by replacing –e from the name of alkane by the suffix – al.
Ketones are named by replacing –e of alkane by the suffix – one.

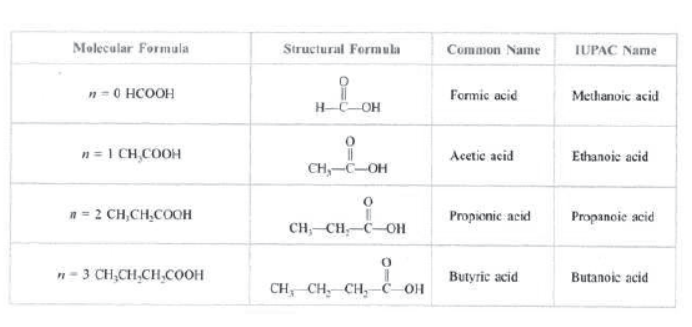
Carboxylic acids: The compounds containing carboxyl (—COOH) group are known as carboxylic acids.
Carboxylic acids are named by substituting e of the corresponding alkane by –oic acid. Their general formula is CnH2n+1—COOH.
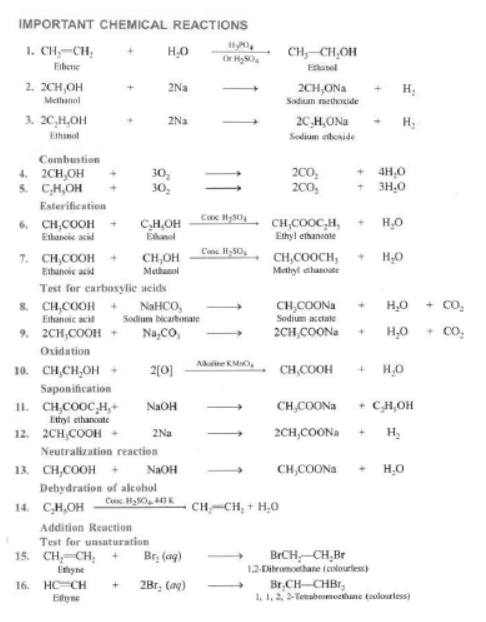
CHEMICL PROPERTIES OF CARBON COMPOUNDS
Combustion of Carbon: Carbon in all allotropic forms burns in presence of oxygen to form carbon dioxide with evolution of heat and light energy. In case of diamond, graphite and fullerene, they burn completely to form CO2 because they are purest form of carbon.
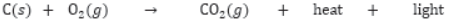
Most of the carbon compounds are combustible and burn in presence of oxygen to form CO2 and H2O, e.g.,
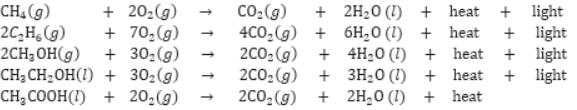
Combustion of Hydrocarbons: If hydrocarbons are burnt in limited supply of oxygen then smoky flame is produced due to incomplete combustion whereas in excess of oxygen, complete combustion takes plane and non-luminous bluish flame with high temperature is produced.
Oxidising Agent: Those substances which can add oxygen to starting material are called oxidising agents, e.g., alkaline KMnO4 and acidified potassium dichromate.
Addition Reactions: Those reactions in which unsaturated compounds, alkenes and alkynes react with a molecule like H2, Cl2, H2O, etc. to form another saturated compounds are called addition reactions. e.g.,
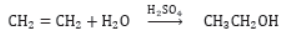
Hydrogenation: It is process in which unsaturated compound reacts with hydrogen in presence of nickel as a catalyst to form saturated compound.
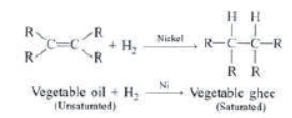
Addition of Hydrogen: Ethyne reacts with hydrogen in the presence of a catalyst to give ethane. Two molecules of hydrogen are added across the carbon-carbon triple bond.
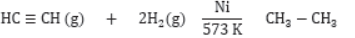
Catalyst: It is a substance which increases the rate of reaction without itself undergoing any permanent chemical change. e.g., Ni, Pt, V2O5 are used as catalyst.
Substitution Reaction: Those reactions in which an atom or group of atoms of a compound is replaced by other atom or group of atoms are called substitution reaction.
Saturated hydrocarbons are less reactive and do not react with most reagents.
They react with halogens in presence of sunlight and undergo substitution reaction. The reaction is very fast.
It is photochemical reaction because it takes place in presence of sunlight.
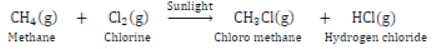
Test for Unsaturation: Add a few drops of bromine water to a test containing ethyne. Shake and observe.
The brown colour of bromine disappears quickly due to the addition of bromine across the carbon-carbon triple bond.
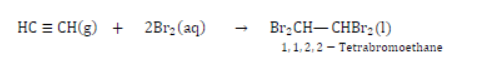
Physical Properties of Ethanol:
I) Pure ethanol is a colour less liquid
II) It has a specific smell and burning taste
III) Its boiling point is 351 K which is higher than corresponding alkanes
IV) It is soluble in water, i.e., it is miscible with water in all proportions
Chemical properties of Ethanol:
I) Dehydration: Ethanol, when heated with conc. H2SO4 at 443 K or Al2O3 at 623 K undergoes dehydration, i.e., loses water molecule to from alkene

II) Reaction with Sodium: Alcohols are very weakly acidic. Ethanol reacts with sodium metal to form sodium ethoxide and hydrogen gas.
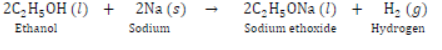
III) Oxidation with Alkaline KMnO4: Ethanol on oxidation with alkaline KMnO4 gives ethanol acid.
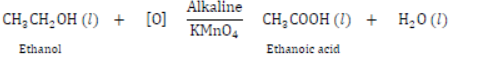
IV) Esterification: Ethanol reacts with Ethanoic acid in presence of concentrated H2SO4 to form ethyl ethanoale and water. The compound formed by the reaction of an alcohol with carboxylic acid is known as ester and the reaction is called Esterification. Esters are sweet fruity smelling compounds because they occur in fruits. They are used in ice creams, cold drinks and perfumes. The reaction takes place as follows.
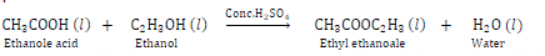
Conc. H2SO4 acts as dehydrating agent, i.e., it removes water formed otherwise formed will get hydrolysed.
V) Combustion: Ethanol is highly inflammable liquid i.e., it catches fire very easily. It burns with blue flame in presence of oxygen to form carbon dioxide and water.

Uses of Ethanol:
I) Ethanol is present in alcoholic beverages such as beer, wine, and whisky.
II) Ethanol is used as antiseptic for sterilizing wounds.
III) Ethanol is used in cough syrups. Digestive syrups and tonics.
IV) Ethanol is being mixed with petrol and is used as motor fuel. This mixture is called power alcohol.
V) A mixture of ethanol and water has lower freezing point than water. This mixture is known as antifreeze and is used in radiators of vehicles in cold countries and at hill stations.
VI) Ethanol is used for preparation of chloroform, iodoform, Ethanoic acid, ethanol, ethyl ethanoate, etc.
VII) Ethyl alcohol is used as hypnotic (induces sleep).
Harmful effects of drinking Alcohol:
I) If ethanol is mixed with CH3OH (methanol) and consumed, it may cause serious poisoning and loss of eyesight.
II) It causes addiction (habit forming) and mixes with blood. It damages liver if taken regularly in large amount.
III) The person loses sense of discrimination under its influence.
IV) Higher amount of consumption of ethanol leads to loss of body control and consciousness. It may even cause death.
Therefore, we should not drink alcohol under any circumstances because it leads to wastage of time, wealth and spoils health.
Alcohol as a Fuel: Alcohol is added to petrol upto 20%. The mixture is called ‘gasol’. It is a cleaner fuel because it creates less pollution. Alcohol, on combustion, gives CO2 and H2O only.
Ethanoic acid (Acetic acid) CH3COOH: Ethanoic acid is most commonly known as acetic acid. Its dilute solution in water (5-8%) is known as vinegar, which is used for preserving food-sausage, pickles etc.
Physical properties:
I) Ethanoic acid is vinegar smelling liquid. The lower carboxylic acids are liquids whereas higher ones are solids.
II) Ethanoic acid is sour in taste. Other lower carboxylic acids are also sour in taste.
III) Ethanoic acid has boiling point 391 K. carboxylic acids have higher boiling points than corresponding alcohols, aldehydes and ketones.
IV) Acetic acid is soluble in water, i.e., it is miscible with water in all proportions. The lower carboxylic acids are soluble in water but solubility in water decreases with increase in molecular weight.
V) Acetic acid freezes at 290 K. thus, in cold weather crystallization of acetic acid may take place that is why pure acetic acid is called glacial acetic acid.
Chemical Properties:
I) Ethanoic acid is weak acid but in turns blue litmus red.
II) Reaction with Metals: Ethanoic acid reacts with metals like Na, K Zn, etc. to form metal ethanoates and hydrogen gas.
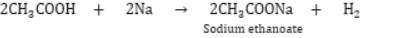
III) Reaction with Carbonates and Bicarbonates: Ethanoic acid reacts with bicarbonates and carbonates and produces brisk effervescence due to formation of carbon dioxide.
IV) Reaction with Base: Ethanoic acid reacts with sodium hydroxide to form sodium ethanoate and water.

Uses of Ethanoic acid:
I) It is used for making vinegar.
II) It is used in making pickles.
Esters: They are pleasant fruity smelling compounds. They are formed by reaction of carboxylic acids and alcohols. They are used in making ice creams, cold drinks, perfumes and in flavoring agents.
Saponification: It is a process in which an ester reacts with sodium hydroxide to form sodium salt of acid and alcohol is formed.
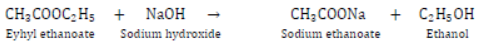
Saponification is also used for preparation of soap.
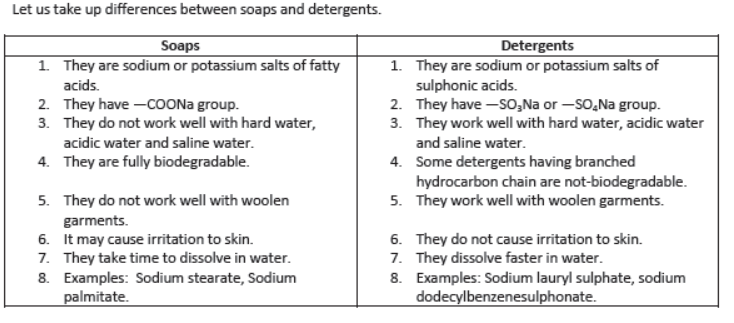
Soaps and Synthetic Detergents:
Soaps: Soaps are sodium or potassium salts of higher fatty acids. Fatty acids are carboxylic acids containing 12 or more carbon atoms, e.g.,
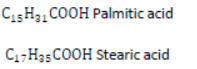
Advantages of Soap:
I) Soap is cheaper and readily available.
II) It works well for cleaning of clothes with soft water (water which does not contain Ca2+ and Mg2+ ).
III) Soaps are 100% biodegradable, i.e., decomposed by microorganisms present in sewage, there, they do not create water pollution.
Disadvantages of Soap:
I) It does not work well with hard water containing Ca2+ and Mg2+ . It reacts with Ca2+ and Mg2+ to form white precipitate which is called scum and soap goes waste. The reaction which takes place is as follows:
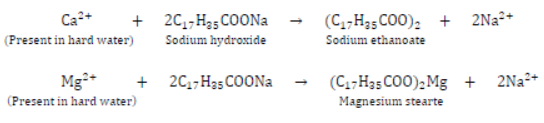
Thus, soap solution forms less lather with hard water.
II) Soaps not suitable for washing woolen garments because it is basic in nature and woolen garments have acidic dyes.
III) Soap is less effective in saline water and acidic water.
Detergents: Detergents are sodium or potassium salts of sulphuric acids of hydrocarbons of alkene type.
They have —SO3Na group or —SO4Na group.
Examples,
I) Sodium lauryl sulphate CH3(CH2)10CH2OSO3Na
II) Sodium dodecylbenzenesulphonate C12H25—C6H4—SO3Na
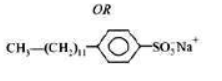
Advantages of Detergents over soaps:
I) Detergents work well even with hard water but soaps do not.
II) Detergents may be used in saline or acidic water.
III) Detergents are more easily soluble in water than soaps.
IV) Detergents can be used for washing woolen garments whereas soaps cannot be used
V) Detergents having linear hydrocarbon chain are biodegradable.
Disadvantages of Detergents over Soaps:
I) Synthetic detergents having branched hydrocarbon chain are not fully biodegradable, i.e., they are not decomposed by microorganisms in sewage and create water pollution.
II) They are more expensive than soaps.
Cleansing Actions of Soaps and Detergents: Soaps and detergents consist of a large hydrocarbon tail with a negatively charged head as shown in figures. The hydrocarbon tail is hydrophobic (water-hating or water repelling) and negatively charged head is hydrophilic (water-loving).
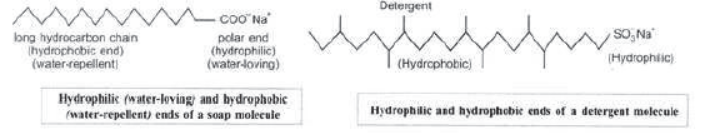
In aqueous solution, water molecules being polar in nature, surround the ions and not the hydrocarbon part of the molecule.
When a soap or detergent is dissolved in water, the molecules associate together as clusters called micelles as shown in figures.
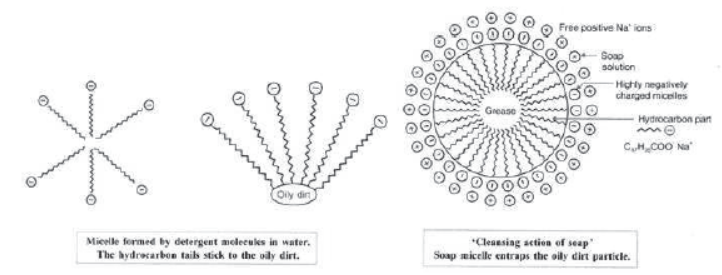
The tails stick inwards and the heads outwards.
In cleansing the hydrocarbon tail attaches itself to oily dirt. When water is agitated (shaken vigorously), the oily dirt tends to lift off from the dirty surface and dissociate into fragments.
This gives opportunity to other tails to stick to oil. The solution now contains small globules of oil surrounded by detergent molecules.
The negatively charged heads present in water prevent in water the small globules from coming together and form aggregates. Thus, the oily is removed.
In the past, detergents caused pollution in rivers and water bodies. The long carbon chain present in detergents used earlier contained lot of branching. These branched chain detergent molecules were degraded very slowly by the microorganisms present in sewage discharge septic tanks and water bodies.
Thus, the detergents persisted in water for long time and made water unfit for aquatic life.
Nowadays, the detergents are made up of molecules in which branching is kept at minimum. These are degraded more easily than branched chain detergents.

Carbon and Its Compounds Class 10 Notes help students to understand the questions asked in the board exam. We have also other study materials for Class 12 like Important Questions, Sample papers, NCERT Solutions, NCERT Books, etc.
We hope you have gotten everything, except assuming you have any issue, you can ask us by writing a comment below with the goal that We can take care of your concerns. A few seconds ago you have perused the Carbon and Its Compounds Class 10 Notes. Here we also gave you the Carbon and Its Compounds Class 10 MCQ Pdf that is not difficult to learn and understand.
Assuming you need to find out with regards to some other study material, then, at that point, you can visit our study material sections.


