Periodic Classification of Elements Class 10 Science Important Questions
Please refer to Periodic Classification of Elements Class 10 Science Important Questions with answers below. These solved questions for Chapter 5 Periodic Classification of Elements in NCERT Book for Class 10 Science have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 10 Science for all chapters in your textbooks.
Important Questions Class 10 Science Chapter 5 Periodic Classification of Elements
All Periodic Classification of Elements Class 10 Science Important Questions provided below have been prepared by expert teachers of Standard 10 Science. Please learn them and let us know if you have any questions.
ONE MARK QUESTIONS
Question: State the Periodic Law on which the Modern Periodic Table is based.
Answer: Periodic Law on which the Modern Periodic Table is based is termed as Modern Periodic Law. According to Modern Periodic Law—The properties of elements are a periodic function of their atomic numbers.
Question: Give reason: The system of classification into triads was not found to be useful.
Answer: The system of classification into Dobereiner’s triads was not found to be useful as he failed to arrange all the then-known elements in the form of triads of elements having similar chemical properties. He could identify only three triads.
Question: How many metals are present in second period of periodic table?
Answer: Second period has 8 elements. There are only two metals in Second period.
These are: Lithium and Beryllium
Question: Predict the maximum number of valence electrons possible for atoms in the first period of the periodic table.
Answer: Maximum number of valence electrons in 1st period is 2.
Question: Give reason why noble gases are placed in a separate group in the modern periodic table.
Answer: It is because they have their outermost shell completely filled and resemble with each other.
Question: Define Newlands law of octaves.
Answer: Newlands Law of Octaves: When elements are arranged in increasing order of their atomic mass, every eighth element resembles with the first.
Question: Out of Li and K, which will have stronger metallic character and why?
Answer: K will have more metallic character because it can lose electrons easily due to its bigger atomic size and less effective nuclear charge.
Question: An element A has atomic number 16. To which group and period does it belong to?
Answer: A(16) has electronic configuration: 2, 8, 6. It has 6 valence electrons and three shells. It belong to Group 16, 3rd period.
Question: An element has atomic number 17. To which group and period does it belong to?
Answer: X(17): 2,8,7 is the electronic configuration. It has 7 valence electrons. It belongs to 17th group and 3rd period.
Question: An element ‘X’ belongs to the second group of periodic table. What is the formula of its chloride? Answer: Since element X belongs to second group so element X has 2 valence electrons. Hence the formula of its chloride will be XCl2.
Question: State modern periodic law.
Answer:Properties of elements are a periodic function of their atomic number.
Question: Write the formula which determines the maximum number of electrons that the shell of an atom can accommodate.
Answer: 2n2, where ‘n’ represents the number of electronic shell.
Question: The electronic configuration of two elements X and Y are 2, 8, 7 and 2, 8, 8, 3 respectively. Write the atomic numbers of X and Y.
Answer: 17 and 21 respectively.
Question: Out of the three elements P, Q and R, having atomic number 11, 17 and 19 respectively, which two elements will show similar properties and why?
Answer: P(11): 2, 8, 1, Q(17): 2, 8, 7, R(19): 2, 8, 8, 1
‘P’ and ‘R’, because they have the same number of valence electrons.
Question: Write the number of horizontal rows in the modern periodic table. What are these rows called?
Answer:There are 7 horizontal rows. These are called periods.
Question: Explain, why the number of elements in the third period are 8.
Answer: It is because 3rd shell could accommodate a maximum of 18 electrons, but if it is the outermost shell it could not have more than 8 electrons. Therefore, this period has 8 elements.
Question: Name the element having electronic configuration 2, 8, 3. What is its valency?
Answer: Aluminium, its valency is equal to 3, because it lose 3 electrons to become stable.
Question: P(3), Q(12), R(13), S(20), which two elements have similar chemical properties and why?
Answer:
P(3): 2, 1; Q(12): 2, 8, 2; R(13): 2, 8, 3; S(20): 2, 8, 8, 2
‘Q’ and ‘S’ because they have the same number of valence electrons.
Question: Where would you locate the element with electronic configuration: 2, 8 in the modern periodic table?
Answer: It belongs to Group 18 and second period of the periodic table.
Question: Give the number of elements in 2nd and 5th period of modern periodic table.
Answer: 2nd period has 8 elements, 5th period has 18 elements.
Question: A metal ‘M’ belongs to 13th group in the modern periodic table. Write the valency of the metal.
Answer:3
Question: What is the number of valence electrons in the last element of the 3rd period?
Answer:8
Question: An element ‘X’ belongs to the second group of periodic table. What is the formula of its chloride?
Answer:XCl2
Question: An element ‘B’ belongs to the second period and Group 13. Give the formula of its oxide.
Answer:B2O3
Question: 3517Cl and 3717Cl are isotopes of chlorine, would you place them in different slots because their atomic masses are different? Or would you place them in the same position because their chemical properties are the same?
Answer: They will be placed in the same slot.
Question: Is it possible to have an element with atomic number 1.5 placed between hydrogen and helium?
Answer: No, atomic number cannot be in fractions.
Question: If an element ‘X’ is placed in group 14, what will be the formula and nature of bonding of its chloride?
Answer:XCl4, it has covalent bonding.
Question: An element ‘A’ has atomic number 17. To which group and period does it belong?
Answer: Its electronic configuration is 2, 8, 7. It belongs to group 17 and 3rd period.
Question: Find the atomic number of the element whose electronic configuration is 2, 8, 5.
Answer: Its atomic number is equal to 2 + 8 + 5 = 15.
Question: An element ‘A’ has atomic number 16. To which group and period does it belong?
Answer: It belongs to group 16 and third (3rd) period.
Question:Name the scientist who first of all showed that atomic number of an element is a more fundamental property than its atomic mass.
Answer:Henry Moseley
Question: How many metals are present in second period of periodic table?
Answer:There are two metals Lithium (Li) and Beryllium (Be) in second period of periodic table.
Question: To which group and period should hydrogen be assigned?
Answer:It is placed in Group 1 and first period.
Question: Write the name, symbol and electronic configuration of an element X whose atomic number is 11.
Answer:Sodium, Na: 2,8,1
Question: How does electropositive character of elements in a period vary from left to right?
Answer: Electropositive character of elements decreases in a period because effective nuclear charge increases from left to right in a period. Due to this effective nuclear charge, the electronegativity increases and the attraction of nucleus on valence shell electrons also increases. So the tendency of losing electrons decreases. Hence the electropositive character of elements decreases in a period.
Note- Non-metals always gain electrons and metals lose their electrons.
Question: How does the elements having similar properties are placed in the same group or period?
Answer: Elements are placed in groups have same number of valence electrons.
Ex- Elements Na and K of first group have 1 valence electron.
Elements are placed in period have same number of shells.
Ex- Elements C, N and O of second period have K and L shells.
Question: How does non-metallic character vary from left to right in a period?
Answer: Non- metallic character increases from left to right in a period because effective nuclear charge increases from left to right in a period. Due to this effective nuclear charge, the electronegativity increases and the tendency of gaining electrons also increases. So non- metallic character increases from left to right in a period.
Note-
(i) Effective nuclear charge is defined as the charge per unit volume.
(ii) From left to right in a period, the effective nuclear charge increases because charge increases from left to right due to increment of proton. But the size remains same because number of shells remains same from left to right in a period.
(iii) From top to bottom in a group, the effective nuclear charge decreases because charge increases down a group due to increment of proton and the size of elements also increases due to increment in shells.
Question: Which has smaller size; Na or Na+?
Answer: Na+ is smaller than Na because Na+ has less number of electrons and its last electrons are in L shell as compared to Na which has last electron in M shell but both have same number of protons. So the attraction of nucleus on valence electrons become strong in Na+ than Na. Hence the size of Na+ become smaller than Na.
Question: How does metallic character vary down a group?
Answer: Metallic character increases down a group because the size of elements increases down a group due to increment in the shells. So the effective nuclear charge decreases down a group. Due to this effective nuclear charge, the electronegativity decreases and the attraction of nucleus on valence shell electrons also decreases. So the tendency of losing electrons increases. Hence metallic character increases down a group.
Question: List the anomalies of Mendeleev’s periodic table which were removed in Modem Periodic Table.
Answer: Co with higher atomic mass proceeds Ni with lower atomic mass. It was solved because Co has lower atomic number than Ni.
Isotopes should have been given different slots due to different atomic mass, but it is not possible due to same chemical properties. The problem was solved because isotopes have same atomic numbers.
Question: The electronic configuration of two elements A and B are 2, 8, 3 and 2, 8, 7 respectively. Find the atomic number of these elements. State the nature and formula of the compound formed by union of these elements.
Answer: A has 2 + 8 + 3 = 13 as its atomic number B has 2 + 8 + 7 = 17 as its atomic number 60 AB3 is the formula of compound. It is an ionic compound.
Question: Choose from the following: 4Be, 9F, 19K, 20Ca
a. The element having one electron in the outermost shell.
b. Two elements of the same group.
Answer: a. 19K(2,8,8,1) has one valence electron,
b. 4Be(2, 2) and 20Ca(2, 8, 8, 2) belongs to the same group.
Question: An element ‘B’ belongs to the second period and Group 13. Give the formula of its oxide.
Answer: Since element B belongs to 13 group and second period so element B has 3 valence electrons and 2 shells. Hence the oxide of B will be B2O3.
Question: Why is K more reactive than Li?
Answer: K is more reactive than Li because the size of K is bigger than Li and due to this bigger size, the effective nuclear charge decreases so the attraction of nucleus on valence electrons also decreases. So K loses its valence electrons more easily than Li.
Question:Why He, Ne and Ar called inert gas?
Answer: He, Ne and Ar are called inert gases because they have complete octet and due to this complete octet they do not react to any other.
Question: Which one has larger atomic size– Cl or Br? Why?
Answer: Br has larger atomic size than Cl because the atomic size increases from top to bottom in a group. From top to bottom in a group, the number of shells increases. So, the atomic size increases.
Question: For the main groups of periodic table given as follows:

(a) Which element is the most metallic?
(b) Which atom is the largest?
Answer: (a) We know that metallic character decreases from left to right in a period and metallic character increases down a group. So element C is the most metallic.
(b) We know that the size of atom decreases from left to right in a period and size of atom increases down a group. So atom C is the largest.
Question: Give the symbols for:
(i) A metal of group 2
(ii) A metal of group 13
(iii) Two non-metals of group 16
(iv) Most reactive non-metal group 17.
Answer: (i) Mg.
(ii) Al.
(iii) O and S.
(iv) Most reactive non-metal of group 17 is fluorine because its electronegativity is more. Its symbol is F.
THREE MARKS QUESTIONS
Questions: State the Modern Periodic Law. What is the number of groups and periods in the Modern Periodic Table?
Answer: The Modern Periodic Law states that ‘properties of elements are a periodic function of their atomic number.’ The number of groups in the Modern Periodic Table are 18 and the number of periods are 7
Question: What is periodicity in properties of elements with reference to the Modern Periodic Table? Why do all the elements of the same group have similar properties?
How does the tendency of elements to gain electrons change as we move from left in a period? State the reason of this change.
Answer : The repetition of similar properties after a definite interval is called periodicity of properties.
Tendency to gain electrons increases along a period from left to right because atomic size decreases.
Question: Justify the following with suitable reasons:
a. Cations are smaller than the corresponding atoms.
b. Size of atom increases as we move down the group.
c. Atomic size decreases as we move across a period.
Answer: a. Cations are formed by loss of electrons, therefore effective nuclear charge increases, size of atom decreases.
b. It is because number of shells goes on increasing down the group.
c. It is because effective nuclear charge increases along a period.
Question: What is the number of valence electrons in the last element of the 3rd size?
Answer: The last element of third size means last element of third period and the last element of third period is Argon (Ar) which has 8 valence electrons.
Question: Which has bigger size; Cl or Cl-?
Answer: Cl- has bigger size as compared to Cl because Cl- has more number of electrons than Cl and nucleus can hold less electrons tightly than more electrons. So the attraction on Cl- become less. Hence, the Size of Cl- become bigger than Cl.
Question: Which one has the bigger size?
Na (11) or Cl (17); Cl (17) or F (9)
Answer: (i) Na has bigger size than Cl because effective nuclear charge increases from left to right in a period. Due to this effective nuclear charge, the electronegativity increases and the attraction of nucleus on valence shell electrons also increases. So the size of atom reduces.
(ii) Cl has bigger size than F because number of shells increases down a group. So the size of atom also increases down a group.
Question: Which one has the smaller size?
K (19) or Na (1); B (5) or C (6)
Answer: (i) Na has the smaller size than K because number of shells increases down a group. So the size of atom increases down a group. Hence K is bigger and Na is smaller.
(ii) C has smaller size than B because effective nuclear charge increases from left to right in a period. Due to this effective nuclear charge, the electronegativity increases and the attraction of nucleus on valence shell electrons also increases. So the size of atom reduces. Hence C has smaller size.
Question: Give two examples of elements of Groups 1, 2, 16 and 17.
Answer: The examples of elements are:
Group 1- Sodium (Na)
Potassium (K)
Group 2- Beryllium (Be)
Magnesium (Mg)
Group 16- Oxygen (O)
Sulphur (S)
Group 17- Fluorine (F)
Chlorine (Cl)
FIVE MARKS QUESTIONS
Question: An element X of group-15 exists as diatomic molecule and combines with hydrogen at 773 K in presence of the catalyst to form a compound, ammonia which has a char- acteristic pungent smell.
(A) Identify element X. How many valence electrons does it have?
(B) Draw the electron dot structure of the diatomic molecule of X. What type of bond is formed in it?
(C) Draw the electron dot structure for ammonia and what type of bond is formed in it?
Answer: (A) Element X which is placed in group 1?? must be nitrogen (N). As it exists as diatomic molecule (N2) and combine with hydrogen to form ammonia.
The electronic configuration of nitrogen is 2, 5; hence, it has 5 valence electrons.
(B) Nitrogen has ?? valence electrons, so it requires three more electrons to complete its octet. Hence, it shares three of its electrons with three electrons of the other nitrogen atom to form a diatomic molecule of N2 gas. Thus, three covalent bonds are formed between two nitrogen atoms and each nitrogen atom is left with one lone pair of electrons.
The electron dot structure of nitrogen molecule is given below:

Nitrogen molecule
(C) The chemical formula of ammonia is NH3. Here, center nitrogen atom is bonded with three hydrogen atoms through three single covalent bonds. The electron dot structure for ammonia is as follows:

Question: ‘‘Atomic number of an element is considered to be a more appropriate parameter than its atomic mass for a chemist.’’ Take the example of the element X (atomic number 13) to justify this statement.
Answer: The electronic configuration of element X having atomic number 13 is: 2, 8, 3
It is a metal having valency = 3 (number of valence electrons). It is electropositive in nature and can form X3+ ions.
It is placed in 3rd period and 13th group of the Modern Periodic table.
Formula of its oxide will be X2O3.
Therefore, atomic number of an element is a more fundamental property and prediction of properties of elements could be made more accurately when elements were arranged on the basis of increasing atomic number.
Question: The position of certain elements in the Modern Periodic Table are shown below.
Using the above table answer the following questions giving reasons in reach case:
(A) Which element will form only covalent compounds?
(B) Which element is a non-metal with valency 2?
(C) Which element is a metal with valency 2?
(D) Out of H2C and F which has largest atomic size?
(E) To which family does H, C and F belong?
Answer: (A) Element ‘E‘ will form only covalent compounds.
Explanation: Element ‘E‘ belongs to 14th group and 3rd period. So its electronic
configuration will be 2, 8, 4. It needs 4 more electrons to complete its octet but it would be diffcultfornucleuswith14 protons to hold on to 18 electrons i.e. extra 4 electrons.
It can lose 4 electrons so that its octet is complete but to gain 4 electrone, it requires a lot of energy to remove those 4 extra electrons.
(B) Element which is a non-metal with valency 2 is ‘B‘.
Explanation: ‘B‘ element belongs to 16th
period and second group so its electronic configuration is 2, 6. This element has 6 valence electrons and it needs only 2 electrons to complete its octet. Therefore,
‘B‘ is a non-metal with valency 2
(C) Element which is a metal with valency 2 is D. It belongs to group 2, so as it has 2 valence electrons and valency 2. It can lose 2 valence electrons and valency 2. It can lose valence electrons and becomes cation (electropositive).
(D) Out of H, C and F, F has the largest size.
Explanation: H, C and F elements belong to the same group i.e. 18th group. As we move down in a group, a new shell of electrons is added at each succeeding element. eq.
‘H‘ element has 1 shell i.e. K shell with 2 electrons ‘C‘ element has 2 shells i.e. K, L shells with 2, 8 electrons ‘F‘ element has 2 shells i.e. K, L, M shells with 2, 8, 8 electrons. Thus, as we move down a group, the number of shells increases. As a result, the distance between the nucleus and the last shell (valence shell) increases and the hence the atomic size increases from to P to bottom.
(E) H, C and F belong to the family known as noble gases or inert gases.
Question: Mendeleev predicted the existence of certain elements not known at that time and named two of them as eka-silicon and eka-aluminium.
(A) Name the elements which have taken the place of these elements.
(B) Mention the group and the period of these elements in the modern periodic table.
(C) Classify these elements as metals, non-metals or metalloids.
(D) How many valence electrons are present in each one of them?
Answer: (A) The two elements that have taken the place of eka-silicon and eka-aluminium are germanium (Ge) and gallium (Ga), respectively.
(B) Germanium is placed in group 14 and period 4 in the modern periodic table. Gallium is placed in group 13 and period 4 in the modern periodic table.
(C) Germanium (Ge) is a metalloid. gallium (Ga) is a metal.
(D) Valence electrons in germanium (Ge) are 4. Valence electrons in gallium (Ga) are 3.
Question: Atomic number of a few elements are given below:

10, 20, 7, 14
(A) Identify the elements.
(B) Identify the group number of these
elements in the periodic table.
(C) Identify the periods of these elements in the periodic table.
(D) What would be the electronic configuration for each of these elements?
(E) Determine the valency of these elements.
Answer: (A) Elements — Neon (Ne), calcium (Ca), nitrogen (N), silicon (Si)
(B) Group — 18, 2, 1??, 14
(C) Period — 2, 4 , 2, 3
(D) Electron configuration — (2, 8); (2, 8, 8, 2); (2,5); (2, 8, 4)
(E) Valency — 0, 2, 3, 4
Question: Define atomic size. Give its unit of measurement. In the modern periodic table
what trend is observed in the atomic radius in a group and a period and why is it so?
Answer: Atomic Size: The distance between the centre of the nucleus and the outer most shell which contains electrons is known as atomic size.

Unit of Atomic Size: Atomic size is measured in
angstroms (°A) or in picometers (pm).
1 °A = 10 – 8 cm = 10–10 m
1 pm = 10–12 m
Variations of Atomic radii in a group : On moving down a group, the atomic radii of elements of increases gradually.
When we move from to P top bottom in a group in modern periodic table, the atomic radius increases as a new shell is added at each succeeding element.
Explanation: For example

It is clear that on moving down the group, the atomic size of the alkali metals increases
Lithium is the first element in the group has the smallest size.
Variations of atomic radii in a period: On moving from left to right in a period, the atomic radii decreases.
As we move from left to right in a period, the atomic number of each succeeding element increased by 1 Extra proton to the nucleus increases the nuclear charge by 1 but addition of extra electron takes place in the same shell.
Owing to this increased nuclear charge, the electrons are attracted closer to the nucleus or nuclear charge tends to pull the electrons closer to the nucleus and reduces the size of the atom.
Explanation: for example Period

In the third period, the atomic size decreases from Na to Cl due to increase in nuclear charge tends to pull the electrons closer to the nucleus and reduces the size of the atom.
Question: Calcium is an element with atomic number 20.
(i) Is it a metal or non-metal?
(ii) Is it more reactive than Mg or less?
(iii) What will be its valency?
(iv) What will be the formula of its Chloride?
(v) Will it be larger than K or smaller?
Answer: (i) Ca is a metal because it contains 2 valence electrons. So it will lose these electrons to acquire stable noble gas electronic configuration.
Ca20- 2, 8, 8, 2
(ii) Yes, Ca is more reactive than Mg because the size of Ca is bigger than Mg. Due to this bigger size, the effective nuclear charge decreases so the attraction of nucleus on valence electrons decreases. So Ca will lose its valence electrons more easily than Mg.
(iii) Ca has two valence electrons so its valency will be 2.
(iv) Ca has two valence electrons and it will lose these two electrons so the formula of its chloride will be CaCl2.
(v) Ca is smaller than K because effective nuclear charge increases from left to right in a period. Due to this effective nuclear charge, the electronegativity increases and the attraction of the nucleus on valence shell electrons also increases. So the size decreases.
Question:

(a) Which is the most reactive metal?
(b) Which is the most reactive non-metal?
(c) Name the family of L, Q, R, T
(d) Name one element from each of groups 2, 13 and 15.
Answer: (a) Most reactive metal is Z because size of atom increases down a group and effective nuclear charge decreases. The attraction of nucleus on valence electrons also decreases. So, the tendency of losing electrons increases. Hence reactivity increases.
(b) We know that electronegativity of non-metals increases from left to right in a period due to the increment of effective nuclear charge and decreases down a group due to the increment of size of atom. So, the most reactive non-metal is L.
(c) L, Q, R, T are the elements of the halogen family.
(d) Group 2- Magnesium (Mg)
Group 13- Boron (B)
Group 15- Nitrogen (N)
Question: This question refers to the elements of the periodic table with atomic numbers 3 to 18.
(a) (i) Which of them are noble gases?
(ii) Which of them are halogens?
(iii) Which of them are akali metals?
(iv) Which are the elements with valency 4?
(b) An element with atomic no. 3 combines with another element with atomic number 17; what would be the formula of the compound?
(c) What is the electronic configuration of an element with atomic number 10?
Answer: (a) (i) Elements of 18 groups are noble gases. So Neon (Ne) with atomic number 10 and Argon (Ar) with atomic number 18 are the noble gases because these have complete octet.
(ii) Elements of 17 group are halogens. So Fluorine (F) with atomic number 9 and Chlorine (Cl) with atomic number 17 are halogens.
(iii) Elements of 1 group are alkali metals except hydrogen (H). So Lithium (Li) with atomic number 3 and Sodium (Na) with atomic number 11 are alkali metals.
(iv) Elements with valency 4 are elements of group 14. So Carbon (C) with atomic number 6 and Silicon (Si) with atomic number 14 are elements with valency 4.
(b) Element with atomic number 3 is Li which has 1 valence electron and element with atomic number 17 is Cl has 7 valence electrons. Element with atomic number 17 wants one electron to complete its octet. So the formula will be LiCl.
(c) The electronic configuration of element with atomic number 10 is 10Ne- 2, 8
Question: An element belongs to 4th period and group 17 of the periodic table. Find out:
(a) The number of valence electrons.
(b) Is it a metal or non-metal.
(c) The name of the element.
(d) Formula of its compound with hydrogen.
(e) Electron dot structure of this element with calcium.
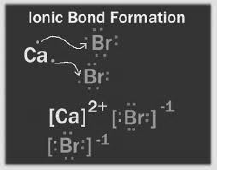
Answer: (a) We know that every element of group 17 has 7 valence electrons. So this element also has 7 valence electrons.
(b) It is non-metal because it can only gain electron.
(c) Its name is Bromine (Br).
(d) The formula of its compound with hydrogen is HBr.
(e)
Question: The position of certain elements in the Periodic Table are shown below. 23
Using the above table answer the following questions:
(a) Which element will form only covalent compounds?
(b) Which element is a non-metal with valency 2?
(c) Which element is a metal with valency 2?
(d) Out of H, C, F, which has largest atomic size?
(e) Give name of family to which H, C and F belong?
Answer: (a) Element E will form only covalent bond because it has 4 valence electrons.
(b) Element B is a non-metal with valency 2 because element B has 6 valence electrons so it can take only two electrons to complete its octet.
(c) Element D is a metal with valency 2 because it has 2 valence electrons.
(d) We know that atomic size increases from up to down in a group due to increment in number of shells. So F has largest atomic size.
(e) Noble gases is the name of family to which H, C and F belong.
Question: Where in period 3 of the modern periodic table do we find:
(a) Non-metals?
(b) Elements forming negative ions?
(c) Elements with high melting points?
(d) Elements forming positive ions?
(e) Metals?
(f) Elements with low boiling points?
Mention their atomic numbers only.
Answer: (a) The elements with atomic number 15, 16, 17 and 18 are non-metals in 3 period because silicon with atomic number 14 is metalloid and rest of the elements are metals in 3 period.
(b) We know that non-metals form negative ions. So, the elements with atomic number 15, 16, 17 and 18 are the elements in 3 period which form negative ions.
(c) We know that metals have highest melting point due to strong metallic bond. So, the elements with atomic number 11, 12 and 13 have highest melting point in 3 period.
(d) We know that metals form positive ions. So, the elements with atomic number 11, 12 and 13 are the elements in 3 period which form positive ions.
(e) The elements with atomic number 11, 12 and 13 are the metals in 3 period.
(f) We know that non-metals have low boiling point. So, the elements with atomic number 15, 16, 17 and 18 are the elements which have low boiling point.
Question: The positions of three elements A, B and C in the periodic table are indicated below:
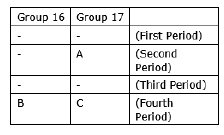
(a) State whether element C would be a metal or a non-metal. Why?
(b) Which is the more active element, A or C? Why?
(c) Which type of ion (cation or anion) will be formed by the element C? Why?
Answer: (a) Element C is non-metal because it belongs to 17 group and it contains 7
valence electrons so it can gain only one electron. So it is non-metal.
(b) In non-metals, electronegativity increases from left to right in a period due to increase in effective nuclear charge and decreases from top to bottom in a group due to increment in size. So A is the most active element.
(c) Since C is non-metal and it has 7 valence electrons. So it can gain only one electron and anion will be formed.
Question: Atoms of seven elements A, B, C, D, E, F and g have a different number of electronic shells but have the same number of electrons in their outermost shells.
The elements A and C combine with chlorine to form and acid and common salt respectively. The oxide of element A is liquid at room temperature and is a neutral substance, while the oxide of the remaining six elements are basic in nature.
Based on the above information, answer the following question:
(i) What could the element A be?
(ii) Will element A to G belong to the same period or same group of the periodic table?
(iii) Write the formula of the compound formed by the reaction of the element A with oxygen.
(iv) Show the formation of the compound by a combination of element C with chlorine with the help of electronic structure.
(v) What would be the ratio of number of combining atoms in a compound formed
by the combination of element A with carbon?
(vi) Which one of the given elements is likely to have the smallest atomic radius?
Answer: (i) A will be hydrogen (H) because A, B, C, D, E, F and G all have same number of valence electrons so this means these elements belong to same group.
Since element C combine to Cl and forms NaCl so these elements belong to 1 group.
Only H form an acid on reaction with Cl in 1 group and its oxide is also neutral substance.
(ii) Elements A to G will belong to same group but periods will different because number of shells are different in all elements.
(iii) A is hydrogen (H) then the formula of the compound formed by the reaction of the element A with oxygen is H2O.
(iv) Element C is Na because C combine with chlorine and forms common salt (NaCl).
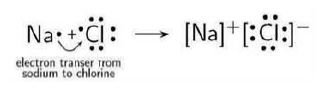
(v) A is H so the compound formed by the combination of element A with carbon will be CH4 and the ratio of combining atoms will be 4:1.
(vi) The element which has the smallest atomic radius is A.
Question: An element has electronic configuration 2, 8, 7.
(a) To which group and period of the long form of Periodic Table does it belong?
(b) What is atomic number of this element?
(c) Is it metallic or non-metallic and why?
(d) Identify the element.
(e) Name an element chemically similar to this element.
Answer: (a) Element belongs to 17 group and 3 period.
(b) Atomic number of this element is 17 because atomic number is equal to total electrons present in it.
(c) It is non-metallic because it has 7 electrons in its valence shell so it can gain only one electron to complete its octet.
(d) This element is Chlorine.
(e) Bromine is chemically similar to this element because bromine and chlorine are the elements of same group.
Question: In the following table, six elements A, B, C, D, E and F (here letters are not the usual symbols of the elements) of the modern Periodic Table with atomic numbers 3 to 18 are given:
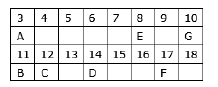
(a) Which of these is (i) a noble gas, (ii) a halogen?
(b) If B combines with F, what would be the formula of the compound formed?
(c) Write the electronic configurations of C and E.
Answer: (a) (i) G with atomic number 10 is a noble gas because it has complete octet.
(ii) F with atomic number 17 is a halogen.
(b) B with atomic number 11 has 1 valence electron and F with atomic number 17 has 7 valence electrons so the formula of the compound formed would be BF.
(c) Electronic configuration of C and E are
E8– 2, 6
C12– 2, 8, 2
Question: (a) How does the atomic radius changes as you go.
(i) From left to right in a period?
(ii) Down a group in the periodic table?
(b) Two elements X and Y have atomic numbers 12 and 16 respectively. Write the electronic configuration for these elements. To which period of the Modern
Periodic Table do these two elements belong? What type of bond will be formed between them and why?
Answer: (a) (i) Atomic radius decreases from left to right in a period because effective nuclear charge increases from left to right in a period. Due to this effective nuclear charge, the electronegativity increases and the attraction of nucleus on valence shell electrons also increases. So the size decreases.
(ii) Atomic radius increases down a group because number of shells increases.
(b) X12– 2, 8, 2
Y16– 2, 8, 6
These two elements belong to 3 period because period of an atom in periodic table is equal to the number of shells present in that atom.
These two elements will form ionic bond because X has two valence electrons so it will completely lose these two electrons to acquire noble gas electronic configuration and Y has six valence electrons and it has more electronegativity so it will gain two electrons to complete its octet. So X will completely give its two electrons to Y and ionic bond will form.
Question: In the following table, are given eight elements A, B, C, D, E, F, G and H (here letters are not the usual symbols of the elements) of the Modern Periodic Table with the atomic numbers of the elements in parenthesis.
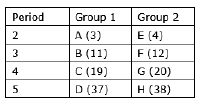
(i) What is the electronic configuration of F?
(ii) What is the number of valence electrons in the atom of F?
(iii) What is the number of shells in the atom of F?
(iv) Write the size of the atoms of E, F, G and H in decreasing order.
(v) State whether F is a metal or a non-metal.
(vi) Out of the three elements B, E and F, which one has the biggest atomic size?
Answer: (i) Electronic configuration of F is F12- 2, 8, 2
(ii) There are 2 valence electrons in the atom of F.
Explanation- Valence electrons are those electrons which present in the last shell.
(iii) There are 3 shells in the atom of F.
(iv) We know that atomic size increases from top to bottom in a group due to increment
in shells. So the order will be H>G>F>E.
(v) Since F has 2 valence electrons so it will donate these two electrons to acquire noble gas electronic configuration. Hence it is a metal.
(vi) We know that atomic size increases from top to bottom in a group and decreases from left to right in a period. So B has the bigger atomic size.
Question: “The atomic number of Lithium is 3.” On the basis of this information
answer the question that follows:
(a) Write the electronic configuration of Li
(b) To which group Li belong?
(c) Find valency of Li.
(d) Identify type of ion it will form.
(e) Write down the formula of the compound formed by it.
Answer: (a) Li3– 2, 1
(b) Li belongs to 1 group.
(c) The valency of Li is 1 because it contains one valence electron.
(d) It will form positive ion because it has one valence electron so it will lose this one
electron to acquire stable noble gas electronic configuration and it will form positive ion.
(e) The formula of the compound formed by it is LiX.
Question: Three elements A, B and C have 3, 4 and 2 electrons respectively in their outermost shell.
Give the group number to which they belong in the modern periodic table. Also, give their valencies.
Answer: The group number for elements can be identified as 10 + number of valence electrons.
Element A with 3 valence electrons will show valency 3 and should belong to group 13. The elements of group 13 are B, Al, Ga, In or Tl.
Element B has 4 valence electrons, so it must be from group 14(10 + 4). The element B can be C, Si, Ge, Sn or Pb.
Similarly, element C has two valence electrons;
hence, it must be from group 2. So element C can be Be, Mg, Ca, Sr, Ba or Ra. 68
VERY SHORT ANSWER TYPE QUESTIONS
Question. How many elements are present in first, second, third and fourth period?
Answer :
| Period number | Shell | Formula | Max.Electron in valence shell | Elements in a period |
| 1. 2. 3. 4. | K L M N | 2n2 2n2 2n2 2n2 | 2 8 8 8 | 2 8 8 18 |
Question. What is the limitation of Döbereiner triads?
Answer : He failed to arrange all the elements in triads having same chemical properties.
Question. What are metalloids? Give 2 examples.
Answer : The elements which show some properties of metal and some properties of non-metal are called semi-metals or metalloids. Example — Boron, Silicon, Germanium, Arsenic.
Question. What was the basis of classification of elements made by Newlands?
Answer : Newlands arranged the elements in the order of increasing atomic masses.
Question. What is the valency of magnesium with atomic number 12 and nitrogen with atomic number 7?
Answer : Magnesium, atomic number = 12
Electronic configuration = 2, 8, 2
∴ Valency = 2
Nitrogen, atomic number = 7
Electronic configuration = 2, 5
∴ Valency = 3
Question. On what basis did Mendeleev classified the element?
Answer : Mendeleev arranged the elements on the basis of their increasing atomic mass and similarity of chemical properties.
Question. How many shells are present in all the elements that belong to period 3?
Answer : All elements in period 3 contain 3 shells in which the electrons are distributed (K, L, M).
Question. Which two chemical properties were considered by Mendeleev for grouping of elements?
Answer : The two chemical properties are:
(a) The nature of compounds formed by elements with oxygen.
(b) The nature of compounds formed by elements with hydrogen.
Question. An element belongs to group 13 and period 3, name the element and give its valency.
Answer : The element is Aluminium.
The valency = 3
Question. State Mendeleev’s Periodic Law.
Answer : The properties of elements are the Periodic Function of their atomic masses.
Question. What is the location of metals and non-metals in the Modern Periodic Table?
Answer : Metals are placed on the left side and non-metals are placed on the right side of the Periodic Table.
Question. Define ‘groups and periods’.
Answer : The vertical columns in a Periodic Table are called groups and the horizontal rows are called periods.
Question. Give one example of Döbereiner’s Triad.
Answer : Li, Na, K
7 23 39
Question. Name three elements discovered later, which filled gaps left by Mendeleev for them.
Answer : Scandium, gallium and germanium.
Question. What are isotopes?
Answer : Isotopes are the atoms of same element having same atomic number but different mass number.
e.g., 12 14
6 C 6 C
1 2 3
1H 1H 1H
Question. How many groups and periods are present in the Modern Periodic Table?
Answer : Modern Periodic Table has 18 groups and 7 periods.
Question. Name two alkali metals present in Group I.
Answer : Alkali metals are Li, Na, K.
Question. Fluorine, chlorine, bromine belong to same group. What is common between them?
Answer : All three elements i.e. fluorine, chlorine, bromine, have same number of valence electrons and same valency.
Question. How does the tendency to lose electrons will change in a period.
Answer : The tendency to lose electrons will decrease across a period as the effective nuclear charge acting on the valence shell electrons increases.
Question. Atomic number of 4 elements is given below which element will belong to the group of Helium.
W X Y Z
8 15 36 20
Answer : Element Y, with atomic number 36 will belong to the same group as He.
Both are inert gas.
atomic number of Y 36 = 2, 8, 18, 8
atomic number of helium 4 = 2, 2
Question. Which is smaller: (i) Na+ or Na, (ii) Cl or Cl– ?
Answer:(i) Na+, (ii) Cl
Question. How does metallic character (electropositive character) varies down the group?
Answer:It increases down the group.
Question. Which has smaller size: K(19) or Na(11); B(5) or C(6)?
Answer:Na(11) is smaller in size than K(19), C(6) is smaller in size than B(5).
Question. How does valency vary in a (i) period on going from left to right, (ii) group?
Answer:(i) In a period, valency first increases till the middle and then it decreases.
(ii) In a group, it remains the same.
Question. On moving from left to right in the second period, what happens to the number of valence electrons?
Answer:Valence electrons keeps on increasing from left to right in the second period.
Question. How does atomic size vary from left to right in a periodic table?
Answer:Atomic size decreases along a period from left to right in the periodic table.
Question. How does reactivity of metals vary down the group?
Answer:It increases down the group.
Question. Give any one difference in the electronic configuration of Group 1 and Group 2 elements.
Answer:Group 1 elements have 1 valence electron and are more reactive than Group 2 elements which have two valence electrons.
Question. Out of Li and K, which one have stronger metallic character and why?
Answer:‘K’, because it can lose electrons easily due to larger size and less effective nuclear charge.
Question. “Fluorine is more electronegative than iodine”. Give reason in support of this.
Answer:‘F’ is smaller in size than I, therefore the tendency to gain electrons is more due to more effective nuclear charge.
Question. List any two properties of the elements belonging to the first group of modern periodic table.
Answer:(i) They should have valency equal to 1 and form monovalent positive ions.
(ii) They are highly reactive soft metals.
Question. The formula of magnesium oxide is MgO. State the formula of barium nitrate and barium sulphate, if barium belongs to the same group.
Answer:Ba(NO3)2, BaSO4
Question. The electronic configuration of two elements ‘A’ and ‘B’ are 2, 8, 7 and 2, 8, 8, 2, respectively. Write the atomic number of these elements. What will be the formula of the compound formed and the nature of bond between them, when these elements chemically combine together?
Answer: A has atomic number ‘17’, ‘B’ has atomic number ‘20’.
BA2 is the formula of the compound. The bond formed between A and B will be ionic bond.
Question. Which has larger atomic radius, K(19) or Ca(20)?
Answer:K(19) is larger than Ca(20).
Question. What would be nature of oxides formed by the elements on the right hand side of periodic table?
Answer:Acidic
Question. Arrange the following metals in decreasing order of atomic size:
Ca, Mg, Ba, Be
Answer:Ba > Ca > Mg > Be
Question. How does valency of an element vary across a period?
Answer:The valency of an element first increases and then decreases across a period.
Question. Define electropositivity.
Answer:It is defined as measure of tendency to lose electrons. The greater the tendency to lose electrons, more will be electropositivity.
Question. The atomic radii of first group elements are given below:
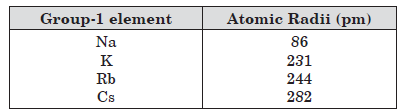
State the reason behind the observed trend in the above elements.
Answer:Atomic radii increases down the group because number of shells go on increasing, effective nuclear charge decreases, distance between nucleus and valence shell increases.
Question. Write the number of valence electrons present in a nitrogen atom(147N) .
Answer: It has 5 valence electrons.
SHORT ANSWER TYPE QUESTIONS
Question. Why Mendeleev could not assign fixed position to hydrogen in the table?
Answer : (a) Hydrogen resembles alkali metal, i.e. like alkali metals it combines with halogen,oxygen and sulphur to form compounds with similar formula as alkalis.
(b) Like halogen, hydrogen also exists as diatomic molecule and combine with metal and non-metals to form covalent compounds.
Question. Name the group number of the following elements, halogens, alkali metals, inert gases, hydrogen, in the Modern Periodic Table.
Answer : Halogens — group No. 17
Alkali metals — group No. 1
Inert gases — group No. 18
Hydrogen — group No. 1
Question. State two characteristics of groups.
Answer : All the elements in a group have the following characteristics:
(a) All element in a group show same number of valence electrons, hence show similar properties.
(b) As we move top to bottom in a group the atomic radius goes on increasing and there is a slight gradation in properties.
Question. What happens to the valency of elements as we move from left to right in a Periodic Table?
Answer : As we move from left to right in a Periodic Table the valency first increases till 4 and then again decreases.
L → R Valency → 1 2 3 4 3 2 1 0 in a period
Question. The number of electrons goes on increasing in the outer shell as we move from left to right in a period, why does the atomic size goes on decreasing?
Answer : In a period all elements have same number of shells. As we move from left to right in a period the number of electrons goes on increasing at the same time the number of
protons also goes on increasing therefore attraction force of nucleus increases and pulls the valence electrons i.e. the outermost shell towards the nucleus and hence the size of atom goes on decreasing.

Size of atom decrease from L → R in a period.
Question. What happens to the metallic character as we move from top to bottom in a group?
Answer : The metallic character increases as we move from top to bottom as the tendency to lose electrons increases.
Question. What happens to the non-metallic character as we move from top to bottom in a group?
Answer : The non-metallic character decreases as we move from top to bottom in a group as the tendency to gain electrons decreases down the group.
Question. The atomic number of ‘X’ is 17. Predict its (a) valency, (b) formula of halide, (c) type of ion formed, (d) reactivity with respect to the other members of same group.
Answer : ‘X’ has atomic number 17
∴ Electronic configuration → 2, 8, 7
(a) Valency = 1
(b) Formula of halide = HX
(c) Type of ion formed = Negative ion (Anion).
(d) Reactivity = Most reactive among those elements which lie below X in a group.
Question. Why are noble gases placed in a separate group?
Answer : All noble gases show same valency i.e. ‘0’, all of them are inert gases, the chemical properties are same and hence they are placed in same group.
Question. Given below are 3 elements W, X, Y and Z the atomic numbers are 9, 10, 16, 17. Predict the following:
(a) Two elements lying in same group.
(b) Element in second period
Answer : (Img 68)
(a) Two elements in same group — W and Z
(b) Element in second period — W and X
Question. State the difference between Modern Periodic Table and Mendeleev’s Periodic Table.
Answer :
| Mendeleev’s Periodic Table | Modern Periodic Table |
| 1. It is based on atomic mass. 2. It has 8 groups and 7 periods. 3. No place for isotopes. | 1. It is based on atomic number. 2. It has 18 groups and 7 periods. 3. Isotopes were not considered. |
Question. Write all the elements present in third period of the Periodic Table and give their electronic configuration.
Answer : Elements of third period are
| Element | Atomic Number | Electronic Configuration | Group No. | Period No. |
| W X Y Z | 9 10 16 17 | 2,7 2,8 2,8,6 2,8,7 | 17 18 16 17 | 2 2 3 3 |
Question. How does electronic configuration helps us to locate the position of element in the Periodic Table?
Answer : The electronic configuration of an atom conveys the valence electrons and number of shells. Valence electrons helps in detecting the group number.
Number of shells in an atom tells the period to which it belongs.
Question. What are the merits of Mendeleev’s Periodic Table?
Answer : Merits of Mendeleev’s Periodic Table are:
(i) Mendeleev left some gaps in his table. Predicted the chemical properties of these 3 elements which were discovered later and had same properties as predicted by Mendeleev, they were gallium, germanium and scandium.
(ii) He arranged the elements very systematically in periods and groups.
LONG ANSWER TYPE QUESTIONS
Question. The atomic number of element X is 17 predict its
(a) Physical state.
(b) Name of element.
(c) Formulae of its compound with hydrogen.
(d) Metal or Non-metal.
(e) Formulae of its molecule.
Answer : Atomic number of X = 17.
Electronic configuration = 2, 8, 7
(a) Physical state → Gas
(b) Chlorine
(c) HCl
(d) Non-metal
(e) Cl2
Question. Two elements A and B belong to group 1 and 2 respectively in the same period. Compare them with respect to:
(a) Valency (b) Size of atom (c) Formula of oxide
(d) Nature of oxide (e) Metallic character
Answer : Group 1 2
Elements A B
(a) Valency A → 1, B → 2
(b) Size of atom → A is bigger atom than B.
(c) Formula of oxide → A2O, BO
(d) Nature → Basic
(e) Metallic character → A is more metallic than B.
Question. Give all characteristics of group.
Answer : Characteristics of a group:
(a) Valence electrons → All elements show same valence electrons in a group.
(b) Valency → Valency of all the elements remains the same in a group.
(c) Atomic size → The atomic size goes on increasing down the group.
(d) Metallic character → In case of metals the metallic character increases down the group.
(e) Non-metallic character → In case of non-metals the non-metallic character decreases down the group.
Question. Give the characteristics of a period.
Answer : In a period as we go from left to right:
(a) Valence electrons → Goes on increasing
1, 2, 3, 4, 5, 6, 7, 8.
(b) Valency → Valency first increases and then decreases
1, 2, 3, 4, 3, 2, 1, 0.
(c) Size of atom → Size of atom goes on decreasing (Img 74)
(d) Metallic character → Decreases
(e) Non-metallic character → Increases
Question. You are given five elements with some description of each element, place them in the Modern Periodic Table.
(a) Essential for breathing and burning.
(b) Inactive, two electrons in the outermost shell.
(c) Atom has same number of protons, electrons and neutrons, used in fertilizer industry.
(d) Number of neutrons, protons are same used in building our bones.
(e) This element form the hardest naturally occurring substance as allotrope.
Answer :
| Element | Period | Group |
| (a) Oxygen (b) Helium (c) Nitrogen (d) Calcium (e) Carbon | 2 1 2 4 2 | 16 18 15 2 14 |
Short Answer Type Questions
Question: Predict the maximum number of valence electrons possible for the elements in the first period of periodic table.
Answer:2 valence electrons are present in the last element ‘Helium’ of 1st period.
Question: Why lithium with atomic number 3 and potassium with atomic number 19 are placed in group one? What will be atomic numbers of first two elements of second group?
Answer: K L M N
Li(3) 2, 1
K(19) 2, 8, 8, 1
Li and K are placed in Group 1 because both have 1 valence electron.
Be(4) and Mg(12) are first two elements of Group 2.
Question: List two anomalies of Mendeleev’s periodic table which were solved by modern periodic table law.
Answer:(i) Position of isotopes were not justified in Mendeleev’s periodic table but it is justified in the modern periodic table.
(ii) Increasing order of atomic masses could not be followed but increasing order of atomic numbers has been followed.
Question: (a) Among the following elements identify the one that would form anions:
K, O, Na, F, Ca, Cl, Hg
(b) Write the electronic configuration of the anions identified above.
Answer:
(a) O, F, Cl will form anions.
(b) O2–(10) 2, 8
F–(10) 2, 8
Cl–(18) 2, 8, 8.
Question: An element belongs to third period and second group of the periodic table:
(a) State number of valence electrons in it.
(b) Is it a metal or non-metal?
(c) Name the element.
(d) Write the formula of its oxide.
Answer:
(a) 2, 8, 2 is the electronic configuration. The number of valence electrons = 2
(b) It is a metal
(c) Magnesium
(d) MgO is the formula of its oxide.
Question: State the reasons for the following:
(a) The elements of the same group have similar chemical properties.
(b) The elements of the same period have different properties.
Answer:
(a) It is because they have the same number of valence electrons.
(b) It is because they differ in the number of valence electrons.
Question: (a) State two main characteristics of elements on which modern periodic table is based.
(b) No fixed position can be assigned to hydrogen in the periodic table. Why?
Answer:
(a) (i) Atomic number, (ii) No. of valence electrons
(b) It is because hydrogen resembles with Group 1 as well as Group 17 elements, therefore no fixed position can be assigned to it.
Question: (a) State modern periodic law.
(b) Elements A, B, C and D have atomic numbers 1, 8, 11 and 19 respectively. Choose the odd element and give reason for your Answer:
Answer:
(a) Modern Periodic Law: It states that the properties of elements are a periodic function of their atomic number.
(b) ‘B’ with atomic number 8 is an odd element because it has 6 valence electrons whereas others have 1 valence electron.
Question: How it can be proved that the basic structure of the Modern Periodic Table is based on the electronic configuration of atoms of different elements?
Answer:Position of element in periodic table is decided with the help of electronic configuration e.g. group number
is decided on the basis of valence electrons e.g., elements having valence electrons 1,2,3,4,5,6,7,8,
belong to Group 1, 2, 13, 14, 15, 16, 17 and 18 respectively.
Period is equal to number of shells e.g. 2,8,3 belong to third period.
Question: The electronic configuration of an element is 2,8,4. State its:
(a) Group and period in the Modern Periodic Table.
(b) Name and write its one physical property.
Answer:
(a) It belongs to Group 14, third period.
(b) Silicon is the element. It is a metalloid, forms covalent bond. It is a semiconductor.
Question: An element ‘X’ has atomic number 13.
(a) Write its electronic configuration.
(b) State the group to which ‘X’ belong.
(c) Is ‘X’ a metal or non-metal?
(d) Write the formula of its bromide.
Answer:
(a) 2, 8, 3, (b) Group 13, (c) Metal, (d) AlBr3
Question: Choose from the following: 6C, 8O, 10Ne, 11Na, 14Si
(a) Elements that should be placed in the same period.
(b) Elements that should be placed in the same group.
State the reason for your selection in each case.
Answer:
(a) 6C, 8O, 10Ne belong to the same period because all these have 2 shells.
11Na, 14Si belong to the same period because both of these have 3 shells.
(b) 6C and 14Si belong to the same group because they have the same number of valence electrons and valency.
Question: The electrons in the atoms of four elements A, B, C and D are distributed in three shells having 1, 3,5 and 7 electrons in the outermost shell respectively. State the period in which these elements can be placed in the modern periodic table. Write the electronic configuration of the atoms A and D and the molecular formula of compound formed when A and D will combine.
Answer:They belong to third period because these have 3 shells.
A has electronic configuration 2, 8, 1, valence electron 1, valency = 1
D has electronic configuration 2, 8, 7, valence electron 7, valency = 1
Formula: AD or A+D–
Question: (a) Predict the following which will form anions and which will form cations:
(i) Na (ii) Al (iii) Cl (iv) O
(b) Name two elements that are inert.
Answer:
(a) Cl and O will form anions
Na and Al will form cations
(b) He, Ne are inert.
Question: An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound.
Answer the following questions giving reason:
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q.
Answer:Atomic number of element P = 20
Electronic configuration of element P = 2, 8, 8, 2
Atomic number of element Q = 17
Electronic configuration of element Q = 2, 8, 7
The position of P in the Modern Periodic Table
Period (Number of shells) = 4
Group (Electrons in outer-most shell) = 2
The position of Q in the Modern Periodic Table
Period (Number of shells) = 3
Group (Electrons in outer-most shell) = (10 + 7) = 17
When P reacts with Q, it loses the two valence electrons (valency 2).
These two valence electrons are accepted by two Q atoms (valency 1).
Hence, the formula of the compound formed between P and Q is PQ2.
Question: From the elements
3919A,2814B , 168C and 4018D identify:
(a) the most electropositive element.
(b) a noble gas.
(c) a metalloid.
(d) an element which will gain 2 electrons to attain nearest noble gas configuration.
(e) formula of compound formed between A and C.
(f) elements belonging to same period.
Answer:(a)3919A is most electropositive element
(b) 4018D is noble gas
(c) 2814B is metalloid
(d) 168Cwill gain 2 electrons to attain nearest noble gas configuration
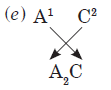
(f) 2814B ,4018Dbelong to same period i.e. 3rd period because they have 3 shells
Question: Name the element which has
(a) the electronic configuration 2, 8, 1
(b) a total of two shells, with 4 electrons in the valence shell.
(c) total of three shells, with 3 electrons in valence shell.
(d) One shell which is completely filled with electrons.
(e) twice as many electrons in the second shell as in the first shell.
Answer:
(a) Sodium (2, 8, 1)
(b) Carbon (2, 4)
(c) Aluminium (2, 8, 3)
(d) Helium (2)
(e) Carbon (2, 4)

