Kinetic Theory MCQ Class 11 Physics
Please refer to Chapter 13 Kinetic Theory MCQ Class 11 Physics with answers below. These multiple-choice questions have been prepared based on the latest NCERT book for Class 11 Physics. Students should refer to MCQ Questions for Class 11 Physics with Answers to score more marks in Grade 11 Physics exams. Students should read the chapter Kinetic Theory and then attempt the following objective questions.
MCQ Questions Class 11 Physics Chapter 13 Kinetic Theory
The Kinetic Theory MCQ Class 11 Physics provided below covers all important topics given in this chapter. These MCQs will help you to properly prepare for exams.
Question. A room temperature the r.m.s. velocity of the molecules of a certain diatomic gas is found to be 1930 m/sec. the gas is
(a) H²
(b) F²
(c) O²
(d) Cl²
Answer
A
Question. Calculate the RMS velocity of molecules of a gas of which the ratio of two specific heats is 1.42 and velocity of sound in the gas is 500 m/s
(a) 727 m/s
(b) 527 m/s
(c) 927 m/s
(d) 750 m/s
Answer
A
Question. One mole of ideal gas required 207 J heat to rise the temperature by 10°K when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by the same 10°K the heat required is (R = 8/3 J/mole °K)
(a) 1987 J
(b) 29 J
(c) 215.3 J
(d) 124 J
Answer
D
Question. One any planet, the presence of atmosphere implies [nrms = root mean square velocity of molecules and ne = escape velocity]
(a) nrms << ne
(b) nrms > ne
(c) nrms = ne
(d) nrms = 0
Answer
A
Question. The r.m.s. speed of the molecules of a gas in a vessel is 200 m/s. if 25% of the gas leaks out of the vessel, at constant temperature, then the r.m.s. speed of the remaining molecules will be
(a) 400 m/s
(b) 150 m/s
(c) 100 m/s
(d) 200 m/s
Answer
D
Question. A unit mass of solid converted to liquid at its melting point. Heat is required for this process is
(a) Specific heat
(b) Latent heat of vaporization
(c) Latent heat of fusion
(d) External latent heat
Answer
C
Question. A gas is taken in a sealed container at 300 K. it is heated at constant volume to a temperature 600 K. the mean K.E. of its molecules is
(a) Halved
(b) Doubled
(c) Tripled
(d) Quadrupled
Answer
B
Question. Energy supplied to convert unit mass of substance from solid to liquid state at its melting point is called
(a) Latent heat of fusion
(b) Evaporation
(c) Solidification
(d) Latent heat of fission
Answer
A
Question. Moon has no atmosphere because
(a) It is far away form the surface of the earth
(b) Its surface temperature is 10°C
(c) The r.m.s. velocity of all the gas molecules is more then the escape velocity of the moons surface
(d) The escape velocity of the moons surface is more than the r.m.s velocity of all molecules
Answer
C
Question. The specific heat of a gas in isothermal process is
(a) Zero
(b) Negative
(c) Remains constant
(d) Infinite
Answer
D
Question. One mole of an ideal gas requires 207 J heat to raise the temperature by 10 K, when heated at constant pressure. If the same gas is heated at constant volume to raise the temperature by 10K, then heat required is
(a) 96.6 J
(b) 124 J
(c) 198.8 J
(d) 215.4 J
Answer
B
Question. 5 gm of air is heated from 273°K to 275°K. the change in internal energy of air will be (CV = 172 cal/kg °K and 4.2 J/cal)
(a) 7.22 J
(b) 5.22 J
(c) 8.16 J
(d) 3.5 J
Answer
A
Question. A cylinder contains 10 kg of gas at pressure of 107 N m–2, the quantity of gas taken out of the cylinder, if final pressure is 2.5 × 106 N m–2 is
(a) 7.5 kg
(b) 10.5 kg
(c) 5.2 kg
(d) 2.5 kg
Answer
A
Question. The average kinetic energy of the molecules of a low density gas at 27°C is
(Boltzmann constant = 1.38 × 10–23 J K–1)
(a) 3.1 × 10–20 J
(b) 3.5 × 10–21 J
(c) 5.3 × 10–18 J
(d) 6.21 × 10–21 J
Answer
D
Question. Pressure versus temperature graph of an ideal gas of equal number of moles of different volumes are plotted as shown in figure. Choose the correct alternative.
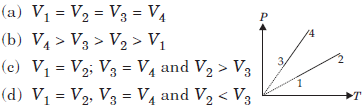
Answer
C
Question. At 27°C temperature, the kinetic energy of an ideal gas is E1. If the temperature is increased to 327°C, then kinetic energy would be
(a) E1/2
(b) E1/√2
(c) √2E1
(d) 2E1
Answer
D
Question. The temperature of the gas contained in a closed vessel increases by 1°C when pressure of the gas is increased by 1%. The initial temperature of the gas is
(a) 100 K
(b) 100°C
(c) 200 K
(d) 250°C
Answer
A
Question. An ideal gas at a pressure of 1 atmosphere and temperature of 27°C is compressed adiabatically until its pressure becomes 8 times the initial pressure. Then the final temperature is {Given ϒ = 3/2}
(a) 627°C
(b) 527°C
(c) 427°C
(d) 327°C
Answer
D
Question. An ideal gas is compressed isothermally until its pressure is doubled and then allowed to expand adiabatically to regain its original volume (ϒ = 1.4 and 2–1.4 = 0.38). The ratio of the final to initial pressure is
(a) 0.76 : 1
(b) 1 : 1
(c) 0.66 : 1
(d) 0.86 : 1
Answer
A
Question. A vessel is filled with a gas at a pressure of 76 cm of mercury of a certain temperature. The mass of the gas is increased by 50% by introducing more gas in the vessel at the same temperature. The resultant pressure of the gas is
(a) 76 cm of mercury
(b) 108 cm of mercury
(c) 112 cm of mercury
(d) 114 cm of mercury
Answer
D
Question. The average kinetic energy of a gas molecule at 27°C is 6.21 × 10–21 J. Its average kinetic energy
at 227°C will be
(a) 52.2 × 10–21 J
(b) 5.22 × 10–21 J
(c) 10.35 × 10–21 J
(d) 11.35 × 10–21 J
Answer
C
Question. The temperature of an ideal gas is increased from 27°C to 127°C, then percentage increase in vrms is
(a) 37%
(b) 11%
(c) 33%
(d) 15.5%
Answer
D
Question. A gas at absolute temperature 300 K has pressure 4 × 10–10 N/m2. The number of molecules per cm3 is of the order of (Boltzmann constant, kB = 1.38 × 10–23 J/K)
(a) 100
(b) 105
(c) 108
(d) 1011
Answer
B
Question. A vessel has 6 g of hydrogen at pressure P and temperature 500 K. A small hole is made in it so that hydrogen leaks out. How much hydrogen leaks out if the final pressure is P/2 and temperature falls to 300 K?
(a) 2 g
(b) 3 g
(c) 4 g
(d) 1 g
Answer
D
Question. One mole of an ideal monoatomic gas at temperature T0 expands slowly according to the law P/V = constant. If the final temperature is 2T0, heat supplied to the gas is
(a) 2RT0
(b) RT0
(c) 3/2 RT0
(d) 1/2 RT0
Answer
A
Question. Given is the graph between PV/T and P for 1 g of oxygen gas at two different temperatures T1 and T2, as shown in figure. Given, density of oxygen = 1.427 kg m–3. The value of PV/T at the point A and the relation between T1 and T2 are respectively
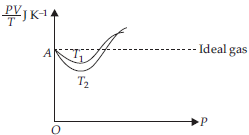
(a) 0.259 J K–1 and T1 < T2
(b) 8.314 J mol–1 K–1 and T1 > T2
(c) 0.259 J K–1 and T1 > T2
(d) 4.28 J K–1 and T1 < T2
Answer
C
Question. 1 mole of H2 gas is contained in a box of volume V = 1.00 m3 at T = 300 K. The gas is heated to a temperature of T = 3000 K and the gas gets converted to a gas of hydrogen atoms. The final pressure would be (considering all gases to be ideal)
(a) same as the pressure initially.
(b) 2 times the pressure initially.
(c) 10 times the pressure initially.
(d) 20 times the pressure initially.
Answer
D
Question. Two containers of equal volume contain the same gas at pressures P1 and P2 and absolute temperatures T1 and T2 respectively. On joining the vessels, the gas reaches a common pressure P and a common temperature T. The ratio P / T is
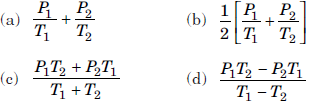
Answer
B
Question. The pressure and density of a diatomic gas (ϒ = 7/5) changes adiabatically from (P, d) to (P′, d ′). If d′/d = 32 then p′/p is
(a) 1/128
(b) 32
(c) 128
(d) 256
Answer
C
Question. A cubic vessel (with faces horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500 m s–1 in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground
(a) remains the same because 500 m s–1 is very much smaller than vrms of the gas.
(b) remains the same because motion of the vessel as a whole does not affect the relative motion of the gas molecules and the walls.
(c) wi l l increase by a factor equal to [v2rms + (500)2]/v2rms, where vrms was the original mean square velocity of the gas.
(d) will be different on the top wall and bottom wall of the vessel.
Answer
B
Question. Two flasks R and S of volume V1 and V2 contain same gas at pressure P1 and P2 respectively at the same temperature. Pressure of the gas when the flasks R and S are connected by a tube of negligible volume is

Answer
A
Question. A vessel has 6 g of oxygen at pressure P and temperature 400 K. A small hole is made in it so that oxygen leaks out. How much oxygen leaks out if the final pressure is P/2 and temperature 300 K?
(a) 5 g
(b) 4 g
(c) 2 g
(d) 3 g
Answer
C
Question. Pressure versus temperature graph of an ideal gas is as shown in figure. Density of the gas at point A is ρ0. Density at point B will be
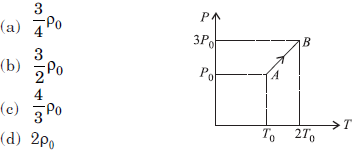
Answer
B
Question. The molecules of a given mass of a gas have root mean square speeds of 100 m s–1 at 27°C and 1 atmospheric pressure. The root mean square speeds of the molecules of the gas at 127°C and 2 atmospheric pressure is
(a) 200/√3
(b) 100/√3
(c) 400/3
(d) 200/3
Answer
A
Question. 1 mole of an ideal gas is contained in a cubical volume V, ABCDEFGH at 300 K as shown in figure. One face of the cube (EFGH) is made up of a material which totally absorbs any gas molecule incident on it. At any given time,

(a) the pressure on EFGH would be zero.
(b) the pressure on all the faces will be equal.
(c) the pressure of EFGH would be double the pressure on ABCD.
(d) the pressure on EFGH would be half that on ABCD.
Answer
D
Question. If CP and CV denoted the specific heats of unit mass of nitrogen at constant pressure and volume respectively, then
(a) CP – CV = R/28
(b) CP – CV = R/7
(c) CP – CV = R/14
(d) CP – CV = R
Answer
A
Question. An inflated rubber balloon contains one mole of an ideal gas, has a pressure P, volume V and temperature T. If the temperature rises to 1.1 T, and the volume is increased to 1.05 V, the final pressure will be
(a) 1.1 P
(b) P
(c) less than P
(d) between P and 1.1
Answer
D
Question. At what temperature is the root mean square speed of an atom in an argon gas cylinder equal to the rms speed of a helium gas atom at –20°C ?
(Atomic mass of Ar 39 u and He = 400 u)
(a) 2.52 × 103 K
(b) 2.52 × 102 K
(c) 4.03 × 103 K
(d) 4.03 ×102 K
Answer
A
Question. The internal energy of one mole of an ideal gas depend upon
(a) Volume of gas
(b) Temperature of gas
(c) Nature of gas
(d) Density of gas
Answer
B
Question. The r.m.s velocity of the molecules of an ideal gas is C at a temperature of 100K. at what temperature is r.m.s. velocity will be doubted?
(a) 200 K
(b) 400 K
(c) 300 K
(d) 50 K
Answer
B
Question. Which of the following is the unit of specific
(a) J kg/°c
(b) J/kg°c
(c) kg °c/J
(d) J kg/°c²
Answer
B
Question. At constant volume temperature is increased then
(a) Collision on walls will be less
(b) Collision frequency will be increases
(c) Collision will be in straight line
(d) Collision will not change
Answer
B
Question. PV/3 = RT, V represents volume of
(a) Any amount of gas
(b) 2 moles of gas
(c) 3 moles of gas
(d) 4 moles of gas
Answer
C
Question. The average kinetic energy of the molecules of a gas at 27°C is 9 10-20 J. what is its average K.E.
at 227°C?
(a) 5 10-20 J
(b) 10 10-20 J
(c) 15 10-20 J
(d) 20 10-20 J
Answer
C
Question. For a gas, the r.m.s. speed at 800K is
(a) Half the value at 200 K
(b) Double the value at 200 K
(c) Same as at 200 K
(d) Four times the value at 200 K
Answer
B
Question. Following gases are kept at the same temperature. Which gas possesses maximum r.m.s. speed?
(a) Oxygen
(b) Nitrogen
(c) Hydrogen
(d) Carbon dioxide
Answer
C

We hope you liked the above Kinetic Theory MCQ Class 11 Physics. In case you have any questions please put them in the comments box below and our teachers will provide you a response.
