Thermodynamics MCQ Class 11 Physics
Please refer to Chapter 12 Thermodynamics MCQ Class 11 Physics with answers below. These multiple-choice questions have been prepared based on the latest NCERT book for Class 11 Physics. Students should refer to MCQ Questions for Class 11 Physics with Answers to score more marks in Grade 11 Physics exams. Students should read the chapter Thermodynamics and then attempt the following objective questions.
MCQ Questions Class 11 Physics Chapter 12 Thermodynamics
The Thermodynamics MCQ Class 11 Physics provided below covers all important topics given in this chapter. These MCQs will help you to properly prepare for exams.
Question. An ideal gas heat engine operates in Carnot cycle between 227°C and 127°C. It absorbs 6 × 104 cal of heat at higher temperature. Amount of heat converted to work is:
(a) 2.4 × 104 cal
(b) 6 × 104 cal
(c) 1.2 × 104 cal
(d) 4.8 × 104 cal
Answer
C
Question. A Carnot engine whose sink is at 300 K has an efficiency of 40%. By how much should the temperature of source be increased, so as to increase its efficiency by 50% of original efficiency?
(a) 275 K
(b) 325 K
(c) 250 K
(d) 380 K
Answer
C
Question. Directions: The following question has four choices out of which ONLY ONE is correct. A refrigerator with its power on, is kept in a closed room with its door open, then the temperature of the room will _________.
(a) rise
(b) fall
(c) remain the same
(d) depend on the area of the room
Answer
A
Question. The translational kinetic energy of gas molecules at temperature T for one mole of a gas is
(a) (3/2) RT
(b) (9/2) RT
(c) (1/3) RT
(d) (5/2) RT
Answer
A
Question. For a diatomic gas change in internal energy for a unit change in temperature for constant pressure and constant volume is U1 and U2 respectively. What is the ratio of U1 and U2?
(a) 5 : 3
(b) 3 : 5
(c) 1 : 1
(d) 5 : 7
Answer
C
Question. A gas is taken through a number of thermodynamic states. What happens to its specific heat?
(a) It is always constant.
(b) It increases.
(c) It decreases.
(d) It can have any value depending upon the process of heat absorbed or evolved.
Answer
D
Question. Which of the following parameters dose not characterize the thermodynamic state of matter?
(a) work
(b) volume
(c) pressure
(d) temperature
Answer
A
Question. A black body at a temperature of 227°C radiates heat at the rate of 20 cal m-2s-1. When its temperature rises to 727°C, the heat radiated will be
(a) 40 units
(b) 160 units
(c) 320 units
(d) 640 units
Answer
C
Question. Directions: The following question has four choices out of which ONLY ONE is correct. A Carnots engine works as a refrigerator between 250 K and 300 K. If it receives 750 calories of heat from the reservoir at the lower temperature, the amount of heat rejected at the higher temperature is __________.
(a) 900 cal
(b) 625 cal
(c) 750 cal
(d) 1000 cal
Answer
A
Question. When a system is taken from state a to state b along the path acb as shown in figure, 60 J of heat flows into the system and 30 J of work is done by the system. Along the path adb, if the work done by the system is 10 J, heat flow into the system is

(a) 100 J
(b) 20 J
(c) 80 J
(d) 40 J
Answer
D
Question. The ideal gas equation for an adiabatic process is
(a) PVϒ = constant
(b) TVϒ+1 = constant
(c) P(ϒ-1)T = constant
(d) Pϒ+1 T = constant
Answer
A
Question. An ideal gas undergoing a change of state from A to B through four different paths I, II, III and IV as shown in the P-V diagram that lead to the same change of state, then the change in internal energy is

(a) is same in I and II but not in III and IV
(b) is same in III and IV but not in I and II
(c) is same in I, II and III but not in IV
(d) same in all the four cases
Answer
D
Question. A system is taken from a given initial state to a given final state along various paths represented on a P-V diagram. The quantity that is independent of the path is
(a) amount of heat transferred Q
(b) amount of work done W
(c) Q but not W
(d) (Q – W)
Answer
D
Question. If an average person jogs, he produces 14.5 × 104 cal min–1. This is removed by the evaporation of sweat. The amount of sweat evaporated per minute (assuming 1 kg requires 580 × 103 cal for evaporation) is
(a) 0.25 kg
(b) 2.25 kg
(c) 0.05 kg
(d) 0.20 kg
Answer
A
Question. Which of the following is not a state function?
(a) Temperature
(b) Entropy
(c) Pressure
(d) Work
Answer
D
Question. A cyclic process is shown in the figure. Work done during the cyclic process ABCDA is

(a) 160 J
(b) 150 J
(c) 600 J
(d) 900 J
Answer
B
Question. In a cyclic process, work done by the system is
(a) zero
(b) more than the heat given to the system
(c) equal to heat given to the system
(d) independent of heat given to the system
Answer
C
Question. Which of the following is not a thermodynamic coordinate?
(a) Gas constant (R)
(b) Pressure (P)
(c) Volume (V)
(d) Temperature (T)
Answer
A
Question. The volume of one mole of an ideal gas changes from V to 2V at temperature 300 K. If R is universal gas constant, then work done in this process is
(a) 300Rln2
(b) 600Rln2
(c) 300ln2
(d) 600ln2
Answer
A
Question. One mole of an ideal gas undergoes a cyclic process ABCD, A as shown in the P-V diagram, The net work done in the process is
(1 atm = 106 dyne cm–2)
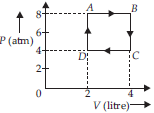
(a) 500 J
(b) 700 J
(c) 800 J
(d) 900 J
Answer
C
Question. In a thermodynamic system working substance is an ideal gas. Its internal energy is in the form of
(a) kinetic energy only
(b) kinetic and potential energy
(c) potential energy
(d) electric energy.
Answer
B
Question. Consider P-V diagram for an ideal gas shown in figure.

Out of the following diagrams, which represents the T-P diagram?

(a) (iv)
(b) (ii)
(c) (iii)
(d) (i)
Answer
C
Question. The change in internal energy of a thermodynamical system which has absorbed 2 kcal of heat and done 400 J of work is (1 cal = 4.2 J)
(a) 2 kJ
(b) 8 kJ
(c) 3.5 kJ
(d) 5.5 kJ
Answer
B
Question. The P-V diagram of a gas undergoing a cyclic process (ABCDA) is shown in the graph, where P is in units of N m–2 and V in cm3. Identify the incorrect statement.
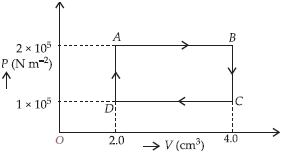
(a) 0.4 J of work is done by the gas from A to B.
(b) 0.2 J of work is done on the gas from C to D.
(c) No work is done by the gas from B to C to C.
(d) Work is done by the gas from B to C and on the gas from D to A.
Answer
D
Question. A sample of ideal gas (g = 1.4) is heated at constant pressure.If 100 J of heat is supplied to the gas the work done by the gas is
(a) 28.57 J
(b) 56.54 J
(c) 38.92 J
(d) 65.38 J
Answer
A
Question. The P-V diagram of path followed by one mole of perfect gas in a cylindrical container is shown in figure, the work done when the gas is taken from state A to state B is

Answer
B
Question. An ideal gas undergoes four different processes from the same initial state as shown in P-V diagram. Out of these four processes which one is isothermal process?
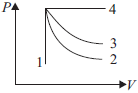
(a) 1
(b) 2
(c) 3
(d) 4
Answer
C
Question. In an adiabatic change the specific heat of a gas is
(a) increase with increase in temperature
(b) decrease with increase in temperature
(c) not depend upon change in temperature
(d) always zero.
Answer
D
Question. The isothermal diagram of a gas at three different temperatures T1, T2 and T3, is shown in the given figure. Then
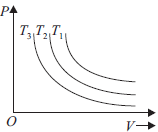
(a) T1 < T2 < T2
(b) T1 < T2 > T2
(c) T1 > T2 > T2
(d) T1 > T2 < T2
Answer
C
Question. For a diatomic gas change in internal energy for a unit change in temperature for constant ressure and constant volume is U1 and U2 respectively. What is the ratio of U1 and U2?
(a) 5 : 3
(b) 3 : 5
(c) 1 : 1
(d) 5 : 7
Answer
C
Question. An ideal gas heat engine operates in a Carnot cycle between 227°C and 127°C. It absorbs 6 kcal at the higher temperature. The amount of heat (in kcal) converted into work is equal to:
(a) 1.6
(b) 1.2
(c) 4.8
(d) 3.5
Answer
B
Question. An engine has an efficiency of 1/6. When the temperature of sink is reduced by 62°C, its efficiency is doubled. Temperature of the source is:
(a) 124°C
(b) 37°C
(c) 62°C
(d) 99°C
Answer
D
Question. A vessel contains a mixture of one mole of oxygen and two moles of nitrogen at 300 K. The ratio of the average rotational kinetic energy per O² to per N² molecule is
(a) 1 : 1
(b) 1 : 2
(c) 2 : 1
(d) depends on the moment of inertia of the two molecules
Answer
A
Question. The temperature of reservoir of Carnots engine operating with an efficiency of 70% is 1000 kelvin. The temperature of its sink is
(a) 300 K
(b) 400 K
(c) 500 K
(d) 700 K
Answer
A
Question. Which of the following statements is correct for any thermodynamic system?
(a) The internal energy changes in all processes.
(b) Internal energy and entropy are state functions.
(c) The change in entropy can never be zero.
(d) The work done in an adiabatic process is always zero.
Answer
B
Question. Directions: The following question has four choices out of which ONLY ONE is correct. Which of the following is incorrect regarding the first law of thermodynamics? A. It is not applicable to any cyclic process B. It is a restatement of the principle of conservation of energy C. It introduces the concept of the internal energy D. It introduces the concept of the entropy
(a) A and D
(b) B and C
(c) C and A
(d) A and B
Answer
A
Question. At a given volume and temperature, the pressure of a gas
(a) varies inversely as its mass
(b) varies inversely as the square of its mass
(c) varies linearly as its mass
(d) is independent of its mass
Answer
C

We hope you liked the above Thermodynamics MCQ Class 11 Physics. In case you have any questions please put them in the comments box below and our teachers will provide you a response.
