Polymers Class 12 Chemistry Important Questions
Please refer to Polymers Class 12 Chemistry Important Questions with answers below. These solved questions for Chapter 15 Polymers in NCERT Book for Class 12 Chemistry have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 12 Chemistry for all chapters in your textbooks.
Important Questions Class 12 Chemistry Chapter 15 Polymers
All Polymers Class 12 Chemistry Important Questions provided below have been prepared by expert teachers of Standard 12 Chemistry. Please learn them and let us know if you have any questions.
Short Answer Questions :
Question. Write the structure of the monomers used for getting the following polymer :
Nylon-6, 6
Answer : Monomers used for getting nylon–6,6, are HOOC(CH2)4COOH (adipic acid) and H2N(CH2)6NH2 (hexamethylenediamine).
Question. What is the primary structural feature necessary for a molecule to make it useful in a condensation polymerisation reaction?
Answer : Monomers should possess more than one functional group.
Question. What is step growth polymserisation? Explain the steps involved in this process.
Answer : Step growth polymerisation involves a repetitive condensation reaction between two bi-functional monomers. Each step produces a distinct functionalised species and in independent of each other.
All condensation polymerisation are step growth polymerisation.
Step : It involves condensation reaction of bifunctional molecules with elimination of smaller molecules like H2O.

Question. (a) Differentiate between copolymerisation and homopolymerisation. Give one example of each.
(b) What is the role of Benzoyl peroxide in preparation of polythene?
Answer : (a) Homopolymer : A polymer made by polymerisation of a single monomer is known as homopolymer and the reaction is called homopolymerisation.
e.g. Polythene made by ethene molecules.
nCH2 = CH2 → (— CH2—CH2 —)n
Ethene Polythene (homopolymer)
Copolymer : A polymer made by polymerisation of two or more different monomers is called copolymer and the reaction is called copolymerisation. When styrene and butadiene are polymerised together, a polymer called styrene-butadiene rubber is formed

(b) In the preparation of polythene from ethene, benzoyl peroxide acts as an initiator or free radical generator.

Question. Draw the molecular structures of the monomers of
(i) PVC
(ii) Teflon
Answer : (i) Structure of monomer PVC n: CH2=CHCl
Vinyl chloride
(ii)
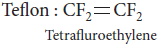
Question. What are biodegradable polymers? Give one example.
Answer : Biodegradable Polymers : The natural polymer, which disintegrates by itself or by micro-ogranisms within certain period of time is called biodegradable polymer, e.g., PHBV (poly–b–hydroxybutyrate–co–b–hydroxyvalerate), Nylon 2–nylon 6.
Question. Draw the structure of the monomers of the following polymers :
(i) Polythene
(ii) PVC
(iii) Teflon
Answer : (i) Polythene : CH2=CH2
Ethene
(ii)

(iii)
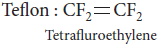
Question. Arrange the following polymers in the increasing order of their intermolecular forces : Polystyrene, Terylene, Buna-S.
Answer : The intermolecular forces are least in case of elastomers like Buna-S while strongest in case of fibres like terylene and in case of thermoplastics like polystrene the intermolecular forces are intermediate in between elastomers and fibres.
Thus, the increasing order of their intermolecular forces is Terylene > Polystyrene > Buna-S
Question. What are biodegradable polymers?
Answer : Biodegradable Polymers : The natural polymer, which disintegrates by itself or by micro-ogranisms within certain period of time is called biodegradable polymer, e.g., PHBV (poly–b–hydroxybutyrate–co–b–hydroxyvalerate), Nylon 2–nylon 6.
Question. ATher the ban on plastic bags, students on one school decided to create awareness among the people about the harmful eftects of plastic bags
Answer : (i) Students show awareness and responsibility towards the environment.
(ii) Biodegradable Polymers : The natural polymer, which disintegrates by itself or by micro-ogranisms within certain period of time is called biodegradable polymer, e.g., PHBV (poly–b–hydroxybutyrate–co–b–hydroxyvalerate), Nylon 2–nylon 6.
(iii) Polythene is homopolymer because it is formed by the repeatition of single monomer unit i.e., ethene, CH2=CH2.
Question. Write the mechanism of free radical polymerisation.
Answer : Chain initiation :

Question. Explain the term co-polymerization and give two examples of copolymers and the reactions for their preparations.
Answer : Copolymerization : When the polymers are synthesised by polymerization of two or more than two different monomers then this process is called as copolymerization. example,
(i) Styrene butadiene rubber (SBR) :

Question. (a) Distinguish between homopolymers and copolymers. Give one example of each.
(b) Is ( CH2—CH(C6H5 )n a homopolymer or a copolymer? Why?
Answer : (a) Homopolymer : A polymer made by polymerisation of a single monomer is known as homopolymer and the reaction is called homopolymerisation.
e.g. Polythene made by ethene molecules.
nCH2 = CH2 → (— CH2—CH2 —)n
Ethene Polythene (homopolymer)
Copolymer : A polymer made by polymerisation of two or more different monomers is called copolymer and the reaction is called copolymerisation. When styrene and butadiene are polymerised together, a polymer called styrene-butadiene rubber is formed

(b) It is a homopolymer because it is formed by the repetition of single compound i.e., monomer unit C6H5CH=CH2.
