Alcohols Phenols and Ethers Class 12 Chemistry Important Questions
Please refer to Alcohols, Phenols and Ethers Class 12 Chemistry Important Questions with answers below. These solved questions for Chapter 11 Alcohols, Phenols and Ethers in NCERT Book for Class 12 Chemistry have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 12 Chemistry for all chapters in your textbooks.
Important Questions Class 12 Chemistry Chapter 11 Alcohols, Phenols and Ethers
All Alcohols, Phenols and Ethers Class 12 Chemistry Important Questions provided below have been prepared by expert teachers of Standard 12 Chemistry. Please learn them and let us know if you have any questions.
Very Short Answer Questions :
Question. Give one chemical test to distinguish between the following pairs of compounds.
1-Propanol and 2-Propanol.
Answer : On adding I2 and NaOH 2-Propanol will give yellow ppt. of iodoform, whereas 1-propanol will not give yellow ppt.
Question. Of the two hydroxy organic compounds ROH and R′OH, the first one is basic and other is acidic in behaviour. How is R different from R′?
Answer : R is alkyl group and R′ is aryl group. R must be a group having more electron density than H. i.e., having +I effect where as R′ must be having –I effect.
Question. How are the following conversions carried out?
(i) Propene to propane-2-ol
(ii) Benzyl chloride to Benzyl alcohol
Answer :

Question. Name the different reagents needed to perform the following reactions :
(i) Phenol to Benzene
(ii) Dehydration of propan-2-ol to propene
(iii) Friedel-Crafts alkylation of anisole
(iv) Dehydrogenation of ethanol to ethanal
Answer : (i) Zinc dust
(ii) Concentrated H2SO4
(iii) Alkyl halide in the presence of anhydrours aluminium chloride, CH3Cl and AlCl3 (anhy.)
(iv) Cu/573 K
Question. Account for the following :
(i) The boiling point of ethanol is higher than that of methanol.
(ii) Phenol is a stronger acid than an alcohol.
Answer : (i) It is due to higher molecular weight, more surface area, more van der Waal’s forces of attraction in C2H5OH than CH3OH.
(ii) Phenols are more acidic than alcohols. It can be explained on the basis that alcohol on losing H+ ions form alkoxide ion and phenol forms phenoxide ion.
The greater acidity of phenol is due to the stability of the phenoxide ion which is resonance stabilized as shown below.

On the other hand, alkoxide ion shows no such resonance stabilisation and is unstable.
Question. How are the following conversions carried out?
(i) Propene to Propan-2-ol
(ii) Ethyl chloride to Ethanal
Answer : (i)

(ii)

Question. Which of the following isomers is more volatile :
o-nitrophenol or p-nitrophenol?
Answer : o-Nitrophenol is more steam volatile than p-Nitrophenol due to the presence of intramolecular H-bonding. p-nitrophenol shows intermolecular H–bonding.

That’s why o-nitrophenol has lower boiling point than p-nitrophenol.
Question. How do you convert the following?
(i) Phenol to anisole
(ii) Propan-2-ol to 2-methylpropan-2-ol
Answer : (i)
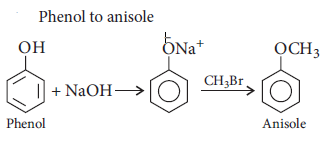
(ii)

Question. Write the structure of the following compound :
2-Methyl-2-ethoxypentane.
Answer :
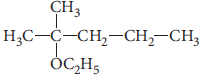
Question. How is toluene obtained from phenol?
Answer :

Question. Give the names of the reagents of bringing about the following transformations :
(i) Hexan-1-ol to hexanal
(ii) But-2-ene to ethanol
Answer : (i) Cu at 573 K
(ii)
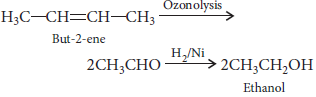
Question. Describe the following with an example :
Kolbe’s reaction
Answer : Kolbe’s reaction : When sodium phenoxide is heated with carbon dioxide under pressure, it gives salicylic acid.

Question. Ortho-nitrophenol has lower boiling point than p-nitrophenol. Why?
Answer : o-Nitrophenol is more steam volatile than p-Nitrophenol due to the presence of intramolecular H-bonding. p-nitrophenol shows intermolecular H–bonding.

That’s why o-nitrophenol has lower boiling point than p-nitrophenol.
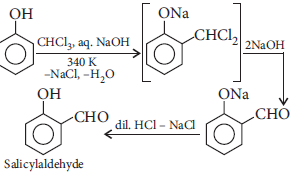
Question. Explain the following with an example for each :
(i) Kolbe’s reaction
(ii) Reimer-Tiemann reaction
Answer : (i) Kolbe’s reaction : When sodium phenoxide is heated with carbon dioxide under pressure, it gives salicylic acid.

(ii) Reimer–Tiemann reaction
Question. How will you convert the following :
(i) Propan-2-ol to propanone.
(ii) Phenol to 2,4,6-tribromophenol.
Answer :

Short Answer Questions :
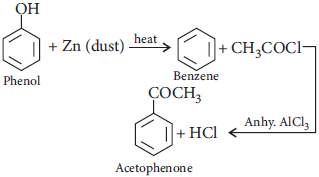
Question. How would you obtain acetophenone from phenol?
Answer :

Question. How would you obtain the following :
(i) 2-methylpentan-2-ol from 2-methyl-1-pentene
(ii) Acetophenone from phenol
Answer : (i)

(ii)

Question. How would you obtain ethane-1, 2-diol from ethanol ?
Answer :

Question. Ortho-nitrophenol is more acidic than orthomethoxyphenol.
Why?
Answer : As we know that the electron withdrawing groups enhance the acidic character of phenols because they help in the stabilisation of phenoxide ion be dispersing negative charge. Nitro group is an electron withdrawing group whereas methoxy group destabiliser the phenoxide ion by intensifying the negative charge. Thus, o-nitrophenol is more acidic than o-methoxyphenol.

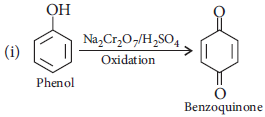
Question. How would you obtain the following :
(i) Benzoquinone from phenol
(ii) 2-Methylpropan-2-ol from methyl magnesium bromide
(iii) Propan-2-ol from propene?
Answer : (i)

(ii)
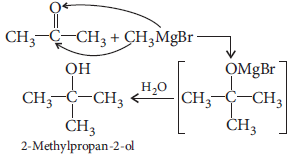
(iii)

Question. (a) Write the mechanism of the following reaction :
CH3CH2OH →HBr CH3CH2Br + H2O
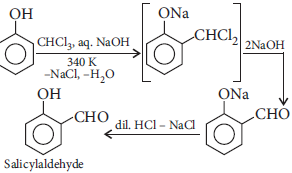
(b) Write the equation involved in Reimer–Tiemann reaction.
Answer : (a) (i) Bromine water, (Br2(aq))
(ii) Lithium aluminium hydride, (LiAlH4) or H2/Ni
(iii) Alkyl halide in the presence of anhydrours aluminium chloride, CH3Cl and AlCl3 (anhy.)
(iv) Acidified potassium permangante, KMnO4,H3O+
(b) Reimer–Tiemann reaction
Question. How will you convert :
(i) Propene to propan-2-ol?
(ii) Phenol to 2,4,6-trinitrophenol?
Answer :
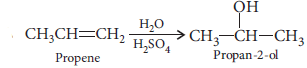
(ii)

Question. Describe a chemical test each to distinguish between the following pairs :
(i) Ethanol and Phenol
(ii) 1-Propanol and 2-Propanol
Answer : (i) Distinction between ethanol and phenol.
FeCl3 test : Phenol gives a violet colouration with FeCl3 solution while ethanol does not.
3C6H5OH + FeCl3 → (C6H5O)3Fe + 3HCl
Phenol Violet colouration
C2H5OH + FeCl3 → No violet colouration.
(ii) On adding I2 and NaOH 2-Propanol will give yellow ppt. of iodoform, whereas 1-propanol will not give yellow ppt.
Question. Write Reimer–Tiemann reaction giving an example.
Answer : Reimer–Tiemann reaction

Question. Explain the following behaviours :
(i) Alcohols are more soluble in water than the hydrocarbons of comparable molecular masses.
(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Answer : (i) The solubility of alcohols in water is due to their ability to form hydrogen bonds with water molecules. Hydrocarbons cannot form such hydrogen bonds, hence they are insoluble in water.
(ii) As we know that the electron withdrawing groups enhance the acidic character of phenols because they help in the stabilisation of phenoxide ion be dispersing negative charge. Nitro group is an electron withdrawing group whereas methoxy group destabiliser the phenoxide ion by intensifying the negative charge. Thus, o-nitrophenol is more acidic than o-methoxyphenol.

Question. Draw the structure of hex-1-en-3-ol compound.
Answer :
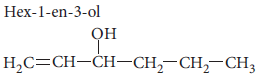
Question. How would you convert ethanol to ethene?
Answer :

Question. The C—O bond is much shorter in phenol than in ethanol. Give reason.
Answer : Due to resonance C—O bond acquires some partial double bond character.
So, in phenol C—O bond length is smaller than ethanol.
Question. Name the reagents used in the following reactions :
(i) Bromination of phenol to 2, 4, 6-tribromophenol
(ii) Butan-2-one to Butan-2-o1
(iii) Friedel–Crafts alkylation of anisole
(iv) Oxidation of primary alcohol to carboxylic acid
Answer : (i) Bromine water, (Br2(aq))
(ii) Lithium aluminium hydride, (LiAlH4) or H2/Ni
(iii) Alkyl halide in the presence of anhydrours aluminium chloride, CH3Cl and AlCl3 (anhy.)
(iv) Acidified potassium permangante, KMnO4,H3O+
Question. Give a separate chemical test to distinguish between the following pairs of compounds :
(i) Ethanol and Phenol
(ii) 2-Pentanol and 3-Pentanol
Answer : (i) Distinction between ethanol and phenol.
FeCl3 test : Phenol gives a violet colouration with FeCl3 solution while ethanol does not.
3C6H5OH + FeCl3 → (C6H5O)3Fe + 3HCl
Phenol Violet colouration
C2H5OH + FeCl3 → No violet colouration.
(ii)
Question. How are the following conversions carried out?
(i) Benzyl chloride to benzyl alcohol.
(ii) Methyl magnesium bromide to 2-methylpropan-2-ol.
Answer : (i)

(ii)
Question. (a) Give chemical tests to distinguish between the following pairs of compounds :
(i) Pentan-2-ol and Pentan-3-ol
(ii) Methanol and Phenol
(b) o-nitro phenol is more acidic than o-methoxy phenol. Explain why.
Answer : (a) (i) On adding I2 and NaOH, 2-pentanol will give yellow precipitate of iodoform whereas 3-pentanol will not give yellow precipitate.

(ii) Distinction between ethanol and phenol.
FeCl3 test : Phenol gives a violet colouration with FeCl3 solution while ethanol does not.
3C6H5OH + FeCl3 → (C6H5O)3Fe + 3HCl
Phenol Violet colouration
C2H5OH + FeCl3 → No violet colouration.
(b) As we know that the electron withdrawing groups enhance the acidic character of phenols because they help in the stabilisation of phenoxide ion be dispersing negative charge. Nitro group is an electron withdrawing group whereas methoxy group destabiliser the phenoxide ion by intensifying the negative charge. Thus, o-nitrophenol is more acidic than o-methoxyphenol.

Question. How are the following conversions carried out?
(i) Benzyl chloride to benzyl alcohol
(ii) Ethyl magnesium chloride to Propan-1-ol
(iii) Propene to Propan-2-ol.
Answer : (i)

(ii) Ethyl magnesium chloride on addition to formaldehyde followed by hydrolysis gives propan-1-ol.

(iii)

Question. Account for the following :
(i) Propanol has higher boiling point than butane.
(ii) Ortho-nitrophenol is more acidic than ortho-methoxyphenol.
Answer : (i) The molecules of butane are held together by weak van der Waals forces of attraction while those of propanol are held together by stronger intermolecular hydrogen bonding.
(ii) As we know that the electron withdrawing groups enhance the acidic character of phenols because they help in the stabilisation of phenoxide ion be dispersing negative charge. Nitro group is an electron withdrawing group whereas methoxy group destabiliser the phenoxide ion by intensifying the negative charge. Thus, o-nitrophenol is more acidic than o-methoxyphenol.

