Biomolecules Class 12 Chemistry Important Questions
Please refer to Biomolecules Class 12 Chemistry Important Questions with answers below. These solved questions for Chapter 14 Biomolecules in NCERT Book for Class 12 Chemistry have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 12 Chemistry for all chapters in your textbooks.
Important Questions Class 12 Chemistry Chapter 14 Biomolecules
All Biomolecules Class 12 Chemistry Important Questions provided below have been prepared by expert teachers of Standard 12 Chemistry. Please learn them and let us know if you have any questions.
Question. Write the name of two monosaccharides obtained on hydrolysis of lactose sugar.
Answer : Lactose on hydrolysis gives b-D-glucose and b-D-galactose.
Question. Where does the water present in the egg go after boiling the egg?
Answer : An egg contains a soluble globular protein called allumin which is present in the white part. On boiling, denaturation (loss of biological activity) of this protein takes place which results in the formation of insoluble fibrous proteins. The water molecules are utilized in this process.
Question. What are enzymes?
Answer : Enzymes : Most of the chemical reactions which occur in living systems process at very slow rates under mild condition of temperature and pH. These reactions are catalysed by a group of biomolecules called enzymes.
Question. Write the main structural difference between DNA and RNA. Of the four bases, name those which are common to both DNA and RNA.
Answer : Structural differences between DNA and RNA
(i) The sugar in DNA is deoxyribose while that in RNA is ribose.
(ii) DNA has a double-stranded helical structure, while RNA has a single-stranded helical structure.
Functional differences between DNA and RNA
(i) DNA is the chemical basis of heredity and is responsible for maintaining the identity of different species.
(ii) RNA molecules are responsible for protein synthesis but the message for the synthesis of a particular protein is present in DNA.
(Structural difference).
Common bases in DNA and RNA are adenine, guanine and cytosine.
Question. Explain the following term :
Polypeptides
Answer : Polypeptides are the macromolecules formed by combination of 10 or more amino acids.
Question. (a) Give two differences between globular and fibrous proteins.
(b) What change occurs in the nature of egg protein on boiling?
Answer : (a) Characteristic differences between globular and fibrous proteins can be given as :

(b) Protein is denatured and its biological activity is lost.
Question. Amino acids show amphoteric behaviour.
Why?
Answer : As amino acids have both acidic (carboxy group) and basic groups (amino group) in the same molecule, they react with both acids and bases. Hence, they show amphoteric behaviour.
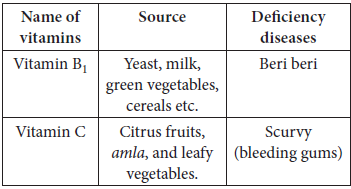
Question. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Answer : Vitamins are classified into two groups depending upon their solubility in water or fat.
(i) Fat soluble vitamins.
(ii) Water soluble vitamins.
Sources of vitamin A : Fish, liver oil, carrots, butter and milk.
Sources of vitamin C : Citrus fruits, amla and green leafy vegetables.
Vitamin K is responsible for the coagulation of blood.
Question. Explain what is meant by a peptide linkage.
Answer : Proteins are the polymers of a-amino acids linked by amide formation between carboxyl and amino group. This is called peptide linkage or peptide bond e.g.,

Question. Define the following term :
Essential amino acids
Answer : Essential amino acids : Amino acids which cannot be synthesized in the body and must be obtained through diet are known as essential amino acids. e.g., valine, leucine, etc.
Question. State two functions of carbohydrates.
Answer : (i) Carbohydrates act as storage molecules as starch in plants and glycogen in animals.
(ii) They act as constituent of cell membrane.
Question. Define the following terms in relation to proteins :
(i) Peptide bond
(ii) Denaturation of proteins
Answer : (i) This is called peptide linkage or peptide bond e.g.,

(ii) Denaturation : The loss of biological activity of a protein by changing the pH, temperature or by adding some salt due to disruption of the native structure of protein is called denaturation.
During denaturation secondary and tertiary structure of protein is destroyed but primary structure remains intact.
Question. Give one example each for fibrous protein and globular protein.
Answer : Globular protein – Insulin
Fibrous protein – Keratin
Question. Explain what is meant by the following :
pyranose structure of glucose?
Answer : The six membered cyclic structure of glucose is called pyranose structure (α-or β –), in analogy with heterocyclic compound pyran.

Question. State what you understand by primary and secondary structure of proteins.
Answer : Primary structure : The specific sequence in which the various amino acids present in a protein are linked to one another is called its primary structure. Any change in the primary structure creates a different protein.
Secondary structure : The conformation of the polypeptide chain is known as secondary structure. The two types of secondary structure are a-helix and b-pleated sheet structure.
In a-helix structure, the polypeptide chain forms all the possible hydrogen bonds by twisting into a right handed screw (helix) with the —NH groups of each amino acid residue hydrogen bonded to the >C=O group of an adjacent turn of the helix. In b-pleated sheet structure, all peptide chains are stretched out to nearly maximum extension and then laid side by side which are held together by intermolecular hydrogen bonds.
Question. Name the three major classes of carbohydrates & give an example of each of these classes.
Answer : On the basis of hydrolysis, carbohydrates can be divided in three major classes :
(i) Monosaccharides : These cannot be hydrolysed into simpler molecules. These are further classified as aldoses and ketoses.
(ii) Oligosaccharides : These are the carbohydrates which on hydrolysis give 2 – 10 monosaccharides. For example, sucrose, lactose, maltose, etc.
(iii) Polysaccharides : These are high molecular mass carbohydrates which give many molecules of monosaccharides on hydrolysis. For example starch and cellulose.
Question. Name the only vitamin which can be synthesized in our body. Name the disease caused due to the deficiency of this vitamin.
Answer : Vitamin D
Disease caused due to deficiency of Vitamin D is rickets.
Question. Write the structural difference between starch and cellulose.
Answer : The basic structural difference between starch and cellulose is of linkage between the glucose units. In starch, there is a-D-glycosidic linkage. Both the components of starch-amylose and amylopectin are polymer of a-D-glucose. On the other hand, cellulose is a linear polymer of b-D-glucose in which C1 of one glucose unit is connected to C4 of the other through b-D-glycosidic linkage.
Question. Define a ‘Peptide linkage’.
Answer : Proteins are the polymers of a-amino acids linked by amide formation between carboxyl and amino group. This is called peptide linkage or peptide bond e.g.,

Question. Shanti, a domestic helper of Mrs. Anuradha, fainted while mopping the floor. Mrs.
Anuradha immediately took her to the nearby hospital where she was diagnosed to be severely ‘anaemic’. The doctor prescribed an iron rich diet and multivitamins supplement to her. Mrs. Anuradha supported her financially to get the medicines. after a month, Shanti was diagnosed to be normal.
after reading the above passage, answer the following questions :
(i) What values are displayed by Mrs. Anuradha?
(ii) Name the vitamin whose dificiency causes ‘pernicious anaemia’.
(iii) Give an example of a water soluble vitamin.
Answer : (i) Humanitarian (kindness and caring)
(ii) Vitamin B12
(iii) Examples of water soluble vitamins :
Vitamin B and vitamin C.
Question. Define the following term :
Polysaccharides
Answer : Carbohydrates which yield a large number of monosaccharide units on hydrolysis are called polysaccharides.
Question. Why Vitamin C cannot be stored in our body?
Answer : Vitamin C is soluble in water and regularly excreted in urine and hence cannot be stored in body.
Question. What is difference between a nucleoside and nucleotide?
Answer : Nucleoside contains pentose sugar, and base whereas nucleotide contains pentose sugar, base as well as phosphate group.
Nucleoside = Base + Sugar
Nucleotide = Base + Sugar + Phosphate.
Question. What is the difference between fibrous protein and globular protein?
Answer : Characteristic differences between globular and fibrous proteins can be given as :

Question. Name one of the water soluble vitamin which is powerful antioxidant. Give its one natural source.
Answer : Vitamin C is water soluble and powerful antioxidant. Natural source of vitamin C is amla.
Question. What type of linkage is present in nucleic acids?
Answer : Ester linkage
Question. What type of linka ge is responsibl e for the formation of proteins?
Answer : Peptide linkage.
Question. Write the product formed on reaction of D-glucose with Br2 water.
Answer : D – Glucose gets oxidised to six carbon carboxylic acid (gluconic acid) on reaction with bromine water.
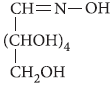
Question. Mention the type of linkage responsible for the formation of the following :
(i) Primary structure of proteins
(ii) Cross-linkage of polypeptide chains
(iii) a-helix formation
(iv) b-sheet structure
Answer : (i) Primary structure – Peptide bond of protein (linkage)
(ii) Cross linkage of – Hydrogen bonds,
polypeptide chain – disulphide linkage, electrostatic force of attraction
(iii) a-helix formation – Hydrogen bond
(iv) b-sheet structure – Intermolecular hydrogen bonds
Question. What are the different types of RNA found in cells of organisms? State the functions of each type.
Answer : RNA are of three types :
(i) Messenger RNA (m-RNA) : Function as messenger carrying the information in a gene to the protein synthesizing machinery.
Transfer RNA (t-RNA) : They transfer the amino acids from cytoplasm to the protein synthesizing machinery.
(ii) Ribosomal RNA (rRNA) : They associates with a set of proteins to form ribosomes. These complex structures, which physically move among an mRNA molecule, catalyze the assembly of amino acids into protein chains. They also bind t-RNAs and various molecules necessary for protein synthesis.
Question. Why are vitamin A and vitamin C essential for us?
Answer : The deficiency of vitamin A leads to xerophthalmia and night blindness. The deficiency of vitamin C leads to scurvy.
Question. What are vitamins? Deficiency of which vitamin causes
(i) Pernicious anaemia?
(ii) Convulsions?
Answer : Organic compounds required in the diet in small amounts to perform specific biological functions for normal maintainance of optimum growth and health of the organism are called vitamins.
(i) Vitamin B12
(ii) Vitamin B6
Question. Answer the following :
(i) What type of linkage is responsible for the primary structure of proteins?
(ii) Name the location where protein synthesis occurs in our body.
Answer : (i) Peptide linkage.
(ii) Protein synthesis takes place in cytoplasm.
Question. What is essentially the difference between a-form and b-form of glucose? Explain.
Answer : In a-D Glucose, the –OH group at C1 is towards right whereas in b-glucose, the –OH group at C1 is towards left. Such a pair of stereoisomers which differ in the configuration only at C1 are called anomers.

Question. What are essential and non-essential amino acids? Give one example of each type.
Answer : Amino acids which cannot be synthesised in the body and must be obtained through diet are known as essential amino acids, e.g., valine and leucine. There are ten essential amino acids. Amino acids which can be synthesised in the body are known as non-essential amino acids, e.g., alanine and glutamic acids.
