Class 10 Science Sample Paper Term 1 Set A
Please see below Class 10 Science Sample Paper Term 1 Set A with solutions. We have provided Class 10 Science Sample Papers with solutions designed by Science teachers for Class 10 based on the latest examination pattern issued by CBSE. We have provided the following sample paper for Term 1 Class 10 Science with answers. You will be able to understand the type of questions which can come in the upcoming exams.
CBSE Sample Paper for Class 10 Science Term 1 Set A
SECTION – A
1. When a lead salt X is heated in a test tube, brown fumes of a gas Y are emitted, along with lead oxide and oxygen gas.

Identify the lead salt X and brown gas Y and select the row containing the correct substances and the type of reaction involved:

Answer
B
2. Which salt has pH more than 7 in solution form:
(a) Na2CO3
(c) NaCl
(b) CaCO3
(d) CaCl2
Answer
A
3. Arrange the metals Ca, Al, Mg and Zn in decreasing order of their reactivities:
(a) Mg > Ca > Al > Zn
(b) Ca > Mg > Al > Zn
(c) Mg > Ca > Zn > Al
(d) Ca > Mg > Zn > Al
Answer
B
4. Which of the following chemical equations are balanced?
(a) AlCl3(aq) + 3NH4OH(aq) → Al(OH)3(s) + 3NH4Cl(aq)
(b) 2AlCl3(aq) + NH4OH(aq) → 2Al(OH)3(s) + NH4Cl(aq)
(c) AlCl3(aq) + 2NH4OH(aq) → Al(OH)3(s) + 2NH4Cl(aq)
(d) 3AlCl3(aq) + 2NH4OH(aq) → 3Al(OH)3(s) + 2NH4Cl(aq)
Answer
A
5. HCl is a stronger acid than acetic acid because:
(a) HCl turns litmus solution red.
(b) HCl is corrosive in nature, but acetic acid not.
(c) HCl dissociates completely, but acetic acid do not.
(d) HCl and acetic acid are equally stronger.
Answer
C
6. In the reaction between MnO2 and HCl, the processes X and Y have been marked as shown in the figure below.

Identify the processes X and Y and the substances oxidized and reduced from the table below:

Answer
D
7. Respiration is considered as an exothermic process because.
(I) During respiration, digested food is broken down and ATP is released.
(II) During respiration, body temperature is increased.
Select the correct option.
(a) Statement (I) is correct
(b) Statement (II) is correct
(c) Both (I) and (II) are correct
(d) Both (I) and (II) are incorrect
Answer
A
8. An aqueous solution ‘A‘ turns phenolphthalein solution pink. On addition of an aqueous solution ‘B‘ to ‘A‘, the pink colour disappears. The following statement is true for solution ‘A‘ and ‘B‘.
(a) A is strongly basic and B is a weak base.
(b) A is strongly acidic and B is a weak acid.
(c) A has pH greater than 7 and B has pH less than 7.
(d) A has pH less than 7 and B has pH greater than 7.
Answer
C
9. The increase in pH value from 7 to 14 indicates:
(a) Increase in concentration of OH– ions
(b) Increase in concentration of H+ ions
(c) Decrease in concentration of OH– ions
(d) No change in concentration of OH– ions
Answer
A
10. What is the difference between the following two types of reactions ?
(I) AgNO3 + HCl → AgCl + HNO3
(II) Mg + 2HCl → MgCl2 + H2
(a) Reaction I is double displacement reaction. Reaction II is single displacement reaction.
(b) Reaction I is single displacement reaction Reaction II is precipitation reaction.
(c) Both reactions are double displacement reaction.
(d) Both reactions are single displacement reaction.
Answer
A
11. The graph below shows the variation of rate of photosynthesis with light intensity for different levels of carbon dioxide.

After analyzing the graph a student writes the following statements.
(I) The rate of photosynthesis increases linearly with light intensity.
(II) The rate of photosynthesis first increases linearly with increase in light intensity and then becomes a constant.
(III) For a given light intensity, the rate of photosynthesis will be more if carbon dioxide concentration is less.
(IV) For a given light intensity, the rate of photosynthesis does not depend upon the carbon dioxide concentration.
Choose from the following which of the following would be the correct statement(s).
(a) Only I
(b) Only II
(c) Both I and III
(d) Both II and IV
Answer
B
12. What protects the inner lining of stomach from hydrochloric acid?
(a) Muscle
(b) Mucus
(c) Basement membrane
(d) Alkaline solution
Answer
B
13. What will happen to a plant if its xylem is removed?
(a) No conduction of water and minerals
(b) No conduction of organic material
(c) Death of plant would occur
(d) Both (a) and (c)
Answer
D
14. What are the end products of anaerobic respiration in yeast?
(a) CO2 and water
(b) Alcohol and CO2
(c) Alcohol and Water
(d) Oxygen and Water
Answer
B
15. Which is the correct sequence of air passage during inhalation?
(a) Nostrils → Larynx → Pharynx → Trachea → Lungs
(b) Nasal passage → Trachea → Pharynx → Larynx → Alveoli
(c) Larynx → Nostrils → Pharynx → Lungs
(d) Nostrils → Pharynx → Larynx → Trachea → Alveoli’
Answer
D
16. A student performed an activity to understand the role of saliva in digestion. He took two test tubes labelled I and II having starch solution and starch solution with saliva respectively. He then added few drops of iodine to the test tubes.

Select the row containing correct observation from the table below :

Answer
A
17. Magnification produced by a rear view mirror fitted in vehicles:
(a) is less than one
(b) is more than one
(c) is equal to one
(d) can be more than or less than one depending upon the position of the object in front of it.
Answer
A
18. The figure shows a ray of light as it travels from medium A to medium B. The refractive index of the medium B relative to medium A is:

Answer
A
19. Study the table below and select the row that has the incorrect information.

Answer
D
20. Study the graph drawn below between the sine of angle of incidence and sine of angle of refraction and choose the correct statement(s):
(I) The graph proves Snell’s law of refraction.
(II) The ratio of sine of angle of refraction to the sine of angle of incidence is known as the refractive index of second medium with respect to the first.
(III) The refractive index of the second medium with respect to the first is less than 1.
(IV) The value of the refractive index for a given pair of media depends upon the speed of light in the two media.
(a) Only I
(b) Both I and IV
(c) Both II and IV
(d) Both II and III
Answer
B
21. Dispersion of white light by a prism is shown in the diagram below. What should be position of second prism in order to recombine the spectra and yield white light?

Answer
A
22. A student writes a few statements after studying the object distances and image distances of spherical mirrors and lenses.
I. A concave mirror gives real, inverted an same size image if the object is placed at C i.e. centre of curvature.
II. A convex mirror forms a virtual and magnified image of the object for all positions of the object.
III. A convex lens forms a real and highly enlarged image if object is placed at Focus.
IV. A concave lens forms a real and diminished image if object is placed between infinity and optical centre O of the lens.
Choose the correct statement(s) from the following:
(a) I and III
(b) II and IV
(c) II and III
(d) I, III and IV
Answer
A
23. An object is placed at a distance of 10 cm in front of a plane mirror, then the distance of image from mirror is:
(a) 5 cm
(b) 10 cm
(c) 20 cm
(d) 0
Answer
B
24. At noon the sun appears white as
(a) Red light is least scattered
(b) Red light is scattered the most
(c) Blue colour is scattered the most
(d) Blue colour is scattered the least
Answer
D
SECTION – B
25. Match the reaction given in column I with the type of reaction in column II.

(a) (I) – (b); (II) – (d); (III) – (a); (IV) – (c)
(b) (I) – (d); (II) – (c); (III) – (b); (IV) – (a)
(c) (I) – (c); (II) – (a); (III) – (b); (IV) – (d)
(d) (I) – (c); (II) – (d); (III) – (a); (IV) – (b)
Answer
C
26. Plaster of Paris is hardened by:
(a) Combining with water
(b) Due to formation of gypsum
(c) Due to formation of slaked lime
(d) Both (a) and (b)
Answer
D
27. Oxides of which of the following metals are amphoteric oxides?
(I) Fe (II) Al
(III) Zn (IV) Mn
(a) Both (I) and (II)
(b) Both (II) and (III)
(c) Both (I) and (IV)
(d) Both (II) and (IV)
Answer
B
28. Common salt conducts electricity in molten state, because :
(a) Na+ and Cl– free to move in molten state but not in solid state
(b) Na+ and Cl– free to move in solid state but not in molten state
(c) Na+ and Cl– free to move in gaseous state but not in solid state
(d) Na+ and Cl– needs force to move in gaseous state
Answer
A
29. The pH of the gastric juices released during digestion is:
(a) equal to 0
(b) equal to 7
(c) less than 7
(d) more than 7
Answer
C
30. Baking powder is a mixture of:
(a) sodium carbonate and ethanoic acid
(b) sodium hydrogen cabonate and ethanoic acid
(c) sodium carbonate and tartaric acid
(d) sodium hydrogen carbonate and tartaric acid
Answer
D
Question No. 31 to 34 consist of two statements – Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
Options:
(a) Both A and R are true, and R is the correct explanation of A.
(b) Both A and R are true, but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
31. Assertion (A): Hydrogen chloride gas does not change the colour of dry blue litmus paper.
Reason (R): Hydrogen chloride gas dissolves in the water present in wet litmus paper to form H+ ions.
Answer
A
32. Assertion (A): When zinc is added to an aqueous solution of iron (II) sulphate, no change is observed.
Reason (R): Zinc is more reactive than iron.
Answer
D
33. Assertion (A): Human heart does not allow mixing of oxygen rich blood with carbon dioxide rich blood.
Reason (R): Human heart has four chambers.
Answer
A
34. Assertion (A): The stars twinkle while the planets do not.
Reason (R): The stars are much bigger in size than the planets.
Answer
B
35. A metal M does not liberate hydrogen from acids but reacts with oxygen to give a black colour product. The metal M is
(a) zinc
(b) iron
(c) copper
(d) aluminium
Answer
C
36. The part of alimentary canal that receives bile from the liver is:
(a) Oesophagus
(b) Stomach
(c) Small intestine
(d) Large intestine
Answer
C
37. The rate of breathing in aquatic organisms is much faster than terrestrial organisms as:
(a) Amount of dissolved oxygen is quite low as compared to amount of oxygen in air.
(b) Amount of dissolved carbon dioxide is quite low as compared to amount of carbon dioxide in air.
(c) Fishes have gills
(d) Heart of fishes have only two chambers.
Answer
A
38. Which of the following statements are incorrect?
(I) Ventricles have thicker muscular walls than atria.
(II) Ventricles pump blood into various organs
(III) Valves in heart ensure that blood does not flow backwards when atria or ventricle relax.
(IV) Deoxygenated blood is transferred to lungs by left ventricle.
(a) Both (I) and (II)
(b) Both (II) and (III)
(c) Both (II) and (IV)
(d) Both (III) and (IV)
Answer
D
39. What will be the nature of the image for an object placed at infinity?
(a) real and erect
(b) real and inverted
(c) virtual and erect
(d) virtual and inverted
Answer
B
40. Select the correct statement:
(a) The focal length of a convex lens having power + 2D is + 50 cm.
(b) The focal length of a convex lens having power – 2D is + 50 cm.
(c) The focal length of a concave lens having power + 2D is + 50 cm.
(d) The focal length of a concave lens having power – 2D is + 50 cm.
Answer
A
41. The figure given below shows the human excretory system with labels (I) to (IV).
Identify the correct label with its functions
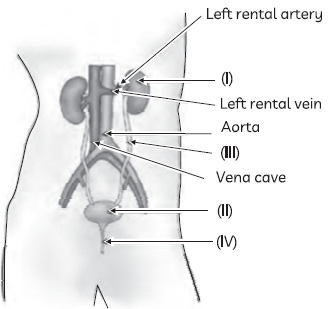
(a) (I) Kidneys: Elimination of unabsorbed food and other wastes
(b) (II) Urinary bladder: Stores urine temporarily
(c) (III) Urethra: Urine is passed out through it
(d) (IV) Ureter: Transfers liquid waste from the kidneys into the urinary bladder.
Answer
B
42. Heart of which of the following organisms have four chambers?
(a) Giraffe
(b) Fishes
(c) Frog
(d) Lizards
Answer
A
43. Study the figure below and answer the question that follows
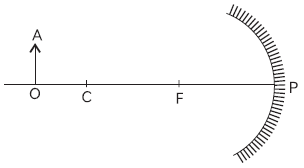
The position and nature of image formed will be:

Answer
A
44. Linear magnification produced by a convex mirror is always positive. This is because:
(a) image formed by a convex mirror is always smaller in size than the object
(b) convex mirror is small mirror
(c) image formed by a convex mirror is always virtual and erect
(d) image formed by convex mirror is real
Answer
C
45. Ritik held a convex lens in his hand and directed it towards the Sun. He focussed the light from the Sun on a sheet of paper to obtain a sharp bright image of the Sun. He hold the paper and the lens in the same position for a while. After a while, he observed that paper begins to burn producing smoke and caught fire after a while.
He wrote the following possible explanation for the case.

(I) image is formed at focus
(II) sun was too bright and temperature was high
(III) The parallel rays of light converged to a point by the lens
(IV) The concentration of the sunlight at apoint generated heat.
(V) The parallel rays of light diverged to a point by the lens.
Select the correct statements w.r.t. to correct explanation.
(a) Statement (I), (II), (III)
(b) Statement (I), (II), (III), (IV)
(c) Statement (I), (III), (IV)
(d) Statement (I), (IV)
Answer
C
46. What is the formula for magnification obtained with a lens?
(a) Ratio of height of image to height of object.
(b) Double the focal length.
(c) Inverse of the radius of curvature.
(d) Inverse of the object distance.
Answer
A
47. An object 4 cm in height, is placed at 15 cm in front of a concave mirror of focal length 10 cm. The distance from the mirror where a screen should be placed to obtain a sharp image of the object is:
(a) 15 cm behind the mirror
(b) 15 cm in front of the mirror
(c) 30 cm behind the mirror
(d) 30 cm in front of the mirror
Answer
D
48. The metals which react with steam but not with hot water is:
(a) Al, Zn, Fe
(b) Pb and Cu
(c) Ag and Au
(d) K, Na, Mg
Answer
A
SECTION – C
Q. 49 to 52 are based on case–1:
Case 1: In the given set up 10 mL of blue coloured coppers sulphate is taken in test tubes A and B. Iron nails are dipped in test tube B for about 20 minutes.
The solution in test tube turns green. Observe the given figure and answer the question that follow.
49. The changes observed in the colour of iron and copper sulphate solution after the reaction shown above are:
(a) colour of iron nail fades and copper sulphate becomes brown
(b) iron becomes brownish and blue colour of copper sulphate fades
(c) iron is unaffected but blue colours of copper sulphate fades
(d) both (a) and (c)
Answer
B
50. The chemical reaction in the above reactions is.
(a) FesO4 + Cu → CuSO4 + Fe
(b) Fe + CuSO4 → FeSO4 + Cu
(c) Fe + CuSO4 → Fe + CuO4 + SO2
(d) Fe + CuSO4 → Cu + FeSO2 + O2
Answer
B
51. Which of the following is more reactive than Cu?
(a) Iron
(b) Silver
(c) Mercury
(d) Platinum
Answer
A
52. The type of reaction involved in the above activity is:
(a) displacement
(b) double displacement
(c) combustion
(d) combination
Answer
A
Q. 53 to 56 are based on case–2:
Case 2: Energy is needed to maintain a state of order in our body. Some organisms use simple food material obtained from inorganic source and other organisms utilise complex substances. These substances have to be broken down into simpler
ones before they can be used for the upkeep and growth of the body.
53. An experiment was conducted to study a factor necessary for photosynthesis.

The test performed on the leaf and the solution used for the test are respectively.
(a) starch test and potassium iodide
(b) chlorophyll test and ethyl alcohol
(c) photosynthesis test and potassium iodide
(d) starch test and ethyl alcohol
Answer
A
54. Which of the following statement(s) is (are) true about stomata?
(I) These are the tiny pores present on the surface of the leaves.
(II) Through these, massive amounts of gaseous exchange take place.
(III) Plants open these pores when carbon dioxide is not required.
(IV) Guard cells operate the opening and closing of these pores.
(a) (I) and (II) only
(b) (I) and (III) only
(c) (I), (II) and (III) only
(d) (I), (II) and (IV) only
Answer
D
55. Study the table below and select the row that has the incorrect information.
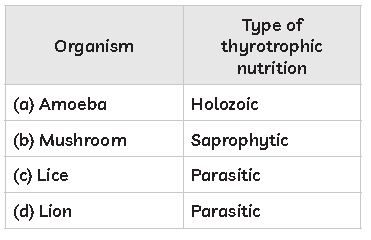
Answer
D
56. Below given diagram represent the cross section of a leaf.

Identify ‘‘P’’ and choose the correct combination of plots provided in the following table.

Answer
B
Q. 57 to 60 are based on case–3:
Case 3: The absolute refractive index of a medium. This ability of medium to refract light is also expressed in terms of its optical density. We have been using rarer medium and ‘denser medium’ which actually means optically rarer medium and optically denser medium. In comparing two media the one with larger refractive index is optically denser and vice versa.

57. In which of the following medium’s does the light travel slowest?
(a) Air
(b) Water
(c) Alcohol
(d) Canada Balsam
Answer
D
58. You are given Dense flint glass, diamond and ice. In which of these does the light travel:
(a) Ice
(b) Dense flint glass
(c) Diamond
(d) Equal in ice and diamond
Answer
A
59. Name the medium having highest optical density in the following:
(a) Benzene
(b) Carbon disulphide
(c) Water
(d) Alcohol
Answer
B
60. Find the medium with lowest optical density in the following:
(a) Air
(b) Fused Quartz
(c) Sapphire
(d) Ruby
Answer
A
