Hydrocarbons MCQ Class 11 Chemistry
Please refer to Chapter 13 Hydrocarbons MCQ Class 11 Chemistry with answers below. These multiple-choice questions have been prepared based on the latest NCERT book for Class 11 Chemistry. Students should refer to MCQ Questions for Class 11 Chemistry with Answers to score more marks in Grade 11 Chemistry exams. Students should read the chapter Hydrocarbons and then attempt the following objective questions.
MCQ Questions Class 11 Chemistry Chapter 13 Hydrocarbons
The Hydrocarbons MCQ Class 11 Chemistry provided below covers all important topics given in this chapter. These MCQs will help you to properly prepare for exams.
Question. Ethylene can be converted into alcohol by treatment with
(a) aq KOH
(b) dil. H2SO4
(c) moist silver oxide
(d) Zn/HCI
Answer
B
Question. The nwnber of optically active products obtained from the complete ozonolysis of the given compound

(a) 0
(b) 1
(c) 2
(d) 4
Answer
A
Question. The test for unsaturation is confinned by the decolourisation of which of the following ?
(a) Iodine water
(b) CuSO4 solution
(c) Bromine water
(d) All of the above
Answer
C
Question. The major product in the reaction of 2-butyne with Li/liq. NH3 is
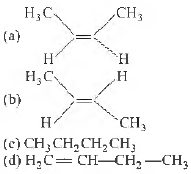
Answer
B
Question. Reaction of one molecule of HBr with one molecule of 1, 3-butadiene at 40°C gives predominantly
(a) 1-bromo-2-butene under kinetically controlled conditions
(b) 3-bromobutene under thermodynamically controlled conditions
(c) 1-bromo-2-butene under thermodynamically controlled conditions
(d) 3-bromobutene under kinetically controlled conditions
Answer
C
Question.

(a) meso diol
(b) racemic diol
(c) Both (a) and (b)
(d) None of the above
Answer
A
Question. Following compound is treated with NBS

Compound formed A is

Answer
B
Question. The Markownikoff’s rule is the best applicable to the reaction between
(a) C2H4 + HCI
(b) C3H6 + Br2
(c) C3H6 + HBr
(d) C3H8 + Cl2
(e) C2H4 + I2
Answer
C
Question. An alkene having molecular formula C9H18 on ozonolysis ives 2, 2-dimethyl propanal and 2-butanone. The alkene is
(a) 2, 2, 2-trimethyl-3-hexene
(b) 2, 2, 6-trirnethyl-3-hexane
(c) 2, 3, 4-trimethyl-2-hexene
(d) 2, 2, 4-trirnethyl-3-hexene
(e) 2, 2-dimethyl-l, 3-heptene
Answer
D
Question. Observe the following reactions and predict the nature of A and B
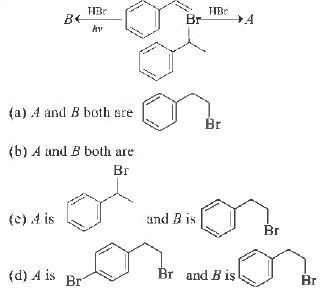
Answer
C
Question. Which of these does not follow anti-Markownikoffs ule?
(a) 2-butene
(b) 1-butene
(c) 2-pentene
(d) 2-hexene
Answer
A
Question. Match the chemical conversion in Column I with the appropriate reagents in Column II and select the correct answer using the codes given below the columns.

Codes
P Q R S
(a) 2 3 1 4
(b) 3 2 1 4
(c) 2 3 4 1
(d) 3 2 4 1
Answer
A
Question. Cycloalkane has the general formula
(a) CnH2n+2
(b) CnH2n-2
(c) CnH2n
(d) C2nH2
(e) C2nH2n
Answer
C
Question. Which of the following cycloalkane gives open chain compound, when reacts with bromine?
(a) cyclopropane
(b) cyclopentane
(c) cyclohexane
(d) cyclooctane
Answer
A
Question. Action of bromine on cyclopentene gives
(a) cyclopentyl bromide
(b) 1, 2-dibromo cyclopentane
(c) cyclopentyl dibromide
(d) No reaction
Answer
B
Question. Cycloalkanes are isomeric with
(a) alkanes
(b) alkenes
(c) alkynes
(d) arenes
Answer
B
Question. Which of the following has the maxinmm heat of hydrogenation ?

Answer
C
Question. A dibromo derivative of an alkane reacts with sodium metal to fonn an cyclic hydrocarbon. The derivative is
(a) 1, 1-dibromopropane
(b) 2, 2-dibromopropane
(c) 1, 2-dibromoethane
(d) 1, 4-dibromobutane
Answer
C
Question. The angle strain in cyclobutane is
(a) 24°44′
(d) 9°44′
(c) 19°22′
(b) 29°16′
Answer
D
Question. Which cycloalkane has the lowest heat of combustion per CH2 group ?
(a) Cyclopropane
(b) Cyclobutane
(c) Cyclopentane
(d) Cyclohexane
Answer
D
Question. Angle strain in cyclopropane is
(a) 24°44′
(b) 9°44′
(c) 44′
(d) – 5°16′
Answer
A
Question. Propyne and propene can be distinguished by
(a) cone. H2SO4
(b) Br2 in CCI4
(c) alk. KMnO4
(d) AgNO3 in NH3
Answer
D
Question. The reactivity of compound Z with clifferent halogens under appropriate conclitions is given below.

The observed pattern of electrophilic substitution can be explained by
(a) the steric effect on the halogen
(b) the steric effect of the tert-butyl group
(c) the electronic effect of the phenolic group
(d) the electronic effect of the tert-buty l group
Answer
A,B,C
Question. For which of the following molecule significantμ μ ≠ 0?

(a) Only r
(b) I and II
(c) Only III
(d) III and IV
Answer
D
Question. Which reagent would you use to carry out the reaction Ethyl benzene ➔ 2 and 4-chloro I-ethyl benzene?
(a) Cl2 light and heat
(b) Cl2 , FeCI3
(c) SOCI2
(d) C2H5 Cl, AlCI3
Answer
B
Question. Which pair is usually required for a nucleophilic aromatic substitution reaction?
(a) An-NO2 substitution and a strong electrophile.
(b) A ring bearing a strong activating group and a strong acid
(c) An aryl halide with an NO2 and astrongnucleophile.
(d) An unsubstituted benzene ring and a strong electrophilic
Answer
C
Question. Which one of the following gives a sooty flame on combustion
(a) C2H4
(b) CH4
(c) C2H6
(d) C6H6
Answer
D
Question. Ethyl benzene cannot be prepared by
(a) Wurtz reaction
(b) Wurtz-Fittig reaction
(c) Friedel-Craft’s reaction
(d) Clemmensen reduction
Answer
A
Question. By passing excess Cl2 (g) in boiling toluene, which one of the following compounds is exclusively fonned
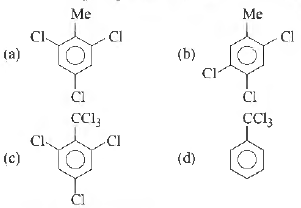
Answer
D
23. Friedel-Craft’s reaction using MeCl and anhydrous AICI3 will take place most efficiently with
(a) benzene
(b) nitrobenzene
(c) acetophenone
(d) toluene
Answer
D
Question. The compounds P , Q and S
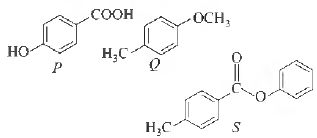
where, separately subjected to nitration using HNO3 / H2SO4 mixture. The major product formed in each case respectively, is
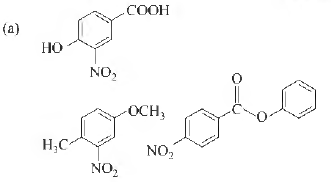
Answer
C
Question. In the reaction,
C6H5CH3 →Oxidation A →NaOH B →Soda lime C
the product C is
(a) C6H5OH
(b) C6H6
(c) C6H5COONa
(d) C6H5ONa
Answer
B
Question. The carbon-carbon bond length in benzene is
(a) in between C2H6 and C2H4
(b) same as in C2H4
(c) in between C2H6 and C2H2
(d) in between C2H4 and C2H2
Answer
A
Question. The carbon-carbon bond distance in benzene is
(a) longer than a C—C single bond
(b) longer than a C = C double bond
(c) shorter than a C = C double bond
(d) shorter than a C = C triple bond
Answer
B
Question. An aromatic compoundX with molecular formula C8H10 produces on nitration one mononitro derivative and three dinitro derivatives.CompoundX would be
(a) ethyl benzene
(b) m-xylene
(c) o -xylene
(d) p-xylene
Answer
D
Question. The overlapping of orbitals in benzene is of the type
(a) sp -sp
(b) p -p
(c) sp2 -sp2
(d) sp3 – sp3
Answer
C
Question. Toluene on treatment with CrO3 and (CH3CO)2O followed by hydrolysis with dil. HCl gives
(a) benzaldehyde
(b) benzoic acid
(c) phenol
(d) phenylacetaldehyde
Answer
A
Question. Which of the following compounds is aromatic?

Answer
D
Question. Which one of the following compounds is prepared in the laboratory from benzene by a substitution reaction?
(a) Glyoxal
(b) Cyclohexane
(c) Acetophenone
(d) Hexabromocyclohexane
Answer
C
Question. Presence of a nitro group in a benzene ring
(a) activates the ring towards electrophilic substitution
(b) renders the ring basic
(c) deactivates the ring towards nucleophilic substitution
(d) deactivates the ring towards electrophilic substitution
Answer
D
Question. The compound formed as a result of oxidation of ethyl benzene by KMnO4 is
(a) benzophenone
(b) acetophenone
(c) benzoic acid
(d) benzyl alcohol
Answer
C
Question. C7H8 →3C12,heat A →Fe/Br2 B →Zn/HCI C Here, the compound C is
(a) 3-bromo -2, 4, 5, 6-trichlorotoluene
(b) o-bromotoluene
(c) p-bromotoluene
(d) m-bromotoluene
Answer
D
Question. Using anhydrous AICl3 as catalyst, which one of the following reactions produce ethylbenzene (PhEt) ?
(a) H3C — CH2OH + C6H6
(b) CH3 — CH = CH2 + C6H6
(c) H2C = CH2 + C6H6
(d) H3C — CH3 + C6H6
Answer
C
Question. Benzene reacts with chlorine in sunlight to give a final product
(a) CCI4
(b) C6H6Cl6
(c) C6Cl6
(d) C6H5CI
Answer
B
Question. A cyclic hydrocarbon molecule has all the carbon and hydrogen in a single plane. All the carbon-carbon bonds are of same length, less than 1.54 A, but more than 1.34 A. The C—C bond angle will be
(a) 109°28′
(b) 100°
(c) 180°
(d) 120°
Answer
D
Question. Pick out the wrong statement.
(a) Toluene shows resonance
(b) The hybrid state of carbon in carbonyl group is sp2
(c) The hyperconjugative effect is known as no bond esonance
(d) Dipole moment of vinyl chloride is less than that of methyl chloride
Answer
B
Question. 4-nitrotoluene →K2Cr2O7 product. The product in the reaction is
H2SO4
(a) benzoic acid
(b) 4-nitrobenzene
(c) 4-nitrobenzoic acid
(d) 2-nitrobenzoic acid
Answer
C
Question. Friedel-Craft acylation can be given by X is

Answer
A
Question. Identify the substitute group, that acts as ortho-para director, during electrophilic substitution in aromatic compounds.
(a) —NH2
(b) —NO2
(c) —SO3H
(d) —N2
Answer
A
Question. According to Huckel’s rule an aromatic compound must possess
(a) (4n + 1) n-electrons
(b) (4n + 2) n-electrons
(c) 4n n-electrons
(d) (4n + 3) n-electrons
Answer
B
Question. Distillation of acetone with concentrated sulphuric acid gives
(a) diacetone alcohol
(b) mesityl oxide
(c) mesitylene
(d) propene-2-ol
Answer
C
Question. Phenyl magnesium bromide reacts with methanol to give
(a) a mixture of anisol and Mg(OH)Br
(b) a mixture of benzene and Mg(OMe)Br
(c) a mixture of toluene and Mg(OH)Br
(d) a mixture of phenol and Mg(Me)Br
Answer
B
Question. Two organic compounds A and B both containing only carbon and hydrogen, on quantitative analysis gave the same percentage composition by weight
C=(12/13)x 100%, H=(1/13)x 100%
A decolourises bromine water but B does not. A and B respectively are
(a) C2H2 and C6H6
(b) C6H6 and C2H2
(c) C2H4 and C2H6
(d) C2H2 and C2H6
Answer
A
Question. What is obtained when chlorine is passed in boiling toluene and product is hydrolysed ?
(a) o-cresol
(b) p-cresol
(c) 2, 4-clihydroxytoluene
(d) benzyl alcohol
Answer
D
Question. Aromatisation of n-heptane by passing over (Al2O3 + Cr2O3 )catalystat773Kgives
(a) benzene
(b) toluene
(c) mixture of both
(d) heptylene
Answer
B
Question. C6H5CH3 →CrO2C12 z
In the given sequence, Z is
(a) benzaldehyde
(c) phenyl acetic acid
(b) toluic acid
(d) benzoic acid
Answer
A
Question. Benzene can be obtained by heating either benzoic acid with X or phenol with Y. X and Y are respectively
(a) zinc dust and soda lime
(b) soda lime and zinc dust
(c) zinc dust and sodium hydroxide
(d) soda lime and copper
Answer
B
Question. Which of the following reacts with benzene in presence of anhydrous aluminium chloride and forms acetophenone ?
(a) CH3CI
(b) CH3COOH
(c) CH3CHO
(d) CH3COCI
Answer
D
Question. The conclitions for aromaticity is
(a) molecule must have clouds of delocalised n-electrons
(b) molecule must contain (4n + 2) n-electrons
(c) Both (a) and (b)
(d) None of the above
Answer
C
Question. Match the following Column I and Column II.
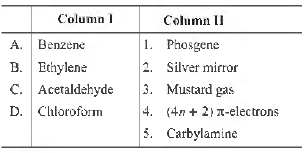
Codes
A B C D
(a) 4 3 2 1
(b) 3 2 1 4
(c) 2 4 5 3
(d) 5 1 4 3
Answer
A
Question. When chlorine is passed through warm benzene in presence of the sunlight, the product obtained iS:
(a) benzotrichloride
(b) chlorobenzene
(c) gammexane
(d) DDT
Answer
C
Question. Aromatic compound among other things should have a 1t-electron cloud containing ( 4n + 2) π-electrons where, n cannot be
(a) 1/ 2
(b) 3
(c) 2
(d) 1
Answer
A
Question. The presence of the chlorine atom on benzene ring makes the second substituent enter at a position
(a) ortho
(b) meta
(c) para
(d) ortholpara
Answer
D
Question. Which of the following have delocalised electron ?
(a) Benzene
(b) Cyclohexane
(c) CH4
(d) C2H6
Answer
A

We hope you liked the above Hydrocarbons MCQ Class 11 Chemistry. In case you have any questions please put them in the comments box below and our teachers will provide you a response.
