Haloalkanes and Haloarenes Class 12 Chemistry Important Questions
Please refer to Haloalkanes and Haloarenes Class 12 Chemistry Important Questions with answers below. These solved questions for Chapter 10 Haloalkanes and Haloarenes in NCERT Book for Class 12 Chemistry have been prepared based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these solved problems properly as these will help them to get better marks in your class tests and examinations. You will also be able to understand how to write answers properly. Revise these questions and answers regularly. We have provided Notes for Class 12 Chemistry for all chapters in your textbooks.
Important Questions Class 12 Chemistry Chapter 10 Haloalkanes and Haloarenes
All Haloalkanes and Haloarenes Class 12 Chemistry Important Questions provided below have been prepared by expert teachers of Standard 12 Chemistry. Please learn them and let us know if you have any questions.
Very Short Answer Questions :
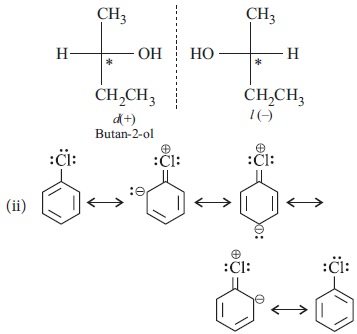
Question. (i) Why is butan-1-ol optically inactive but butan-2-ol is optically active?
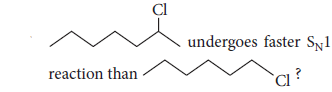
(ii) Although chlorine is an electron withdrawing group, yet it is ortho-, para-directing in electrophilic aromatic substitution reactions. Why?
Answer : (i) Butan-1-ol is achrial, i.e., does not have chiral ‘C’ atom which is attached to four different groups, therefore, it is optically inactive.
CH3—CH2—CH2—CH2OH
Butan-1-ol
(Optically inactive)
(No chiral carbon)
Butan-2-ol is chiral, i.e., has chiral ‘C’ atom, attached to four different groups.
Although Cl is electron withdrawing (I effect) but still o-and p-directing as due to +R effect, electrons density is maximum at o-and p-positions.
Question. A solution of KOH hydrolyses CH3CHClCH2CH3 and CH3CH2CH2CH2Cl.
Which one of these is more easily hydrolysed?
Answer : CH3CH2CHCH3 Clhydrolyses easily with KOH because it is secondary halide.
Question. (a) Why is sulphuric acid not used during the reaction of alcohols with KI in the conversion of an alcohol to the alkyl iodide?
(b) Why are haloarenes less reactive than haloalkanes towards nucleophilic substitution reactions?
Answer : (a) H2SO4 is an oxidant. KI reacts with H2SO4 and give HI and H2SO4 oxidises HI to I2.
2KI + H2SO4 → 2KHSO4 + 2HI
2HI + H2SO4 → 2H2O + I2 + SO2
Thus HI will not be available for reaction with alcohol to form alkyl iodide.
This is why sulphuric acid is not used during the reaction of alcohols with KI.
(b) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions due to the following reasons.
(i) Resonance effect : In haloarenes the electron pairs on halogen atom are in conjugation with p-electrons of the ring and the following resonating structures are possible.

C—Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is dificult than haloalkane and therefore, are less reactive towards nucleophilic substitution reaction.
(ii) difference in hybridisation of carbon atom in C—X bond.
Question. Suggest a possible reason for the following observations :
(i) The order of reactivity of haloalkanes is RI > RCl > RBr.
(ii) Neopentyl chloride (CH3)3CCH2Cl does not follow SN2 mechanism.
Answer : (i) Among the various halides with same alkyl group the order of reactivity is RI > RBr > RCl.
Due to increasing bond strength of C—I, C—Br and C—Cl the reactivity decreases.
(ii) Neopentyl chloride being a primary halide reacts slowly through SN1 and the carbon carrying halogen is sterically more hindered. Hence it does not follow SN2 mechanism.
Question. How do you convert?
(i) Chlorobenzene to biphenyl
(ii) 2-bromobutane to but-2-ene
Answer :

Question. State one use each of DDT and iodoform.
Answer : (ii) DDT is used as an insecticide and iodoform is used as a mild antiseptic.
Question. Explain the following reactions with an example :
Friedel-crafts reaction.
Answer : Haloarenes can undergo both freidal craft alkylation (with alkyl halide) or freidal craft acylation (with acid halide) in presence of Lewis acid catalyst to give a mixture of o- and p-haloalkyl benzene or o- and p-haloacylbenzene.

Question. How would you account for the following :
(i) Grignard reagents are prepared strictly under anhydrous conditions ?
(ii)
Answer : (i) Grignard reagents react with water to form alkanes.
R—Mg—X + H2O → R—H + Mg<XOH
So, they must be prepared under anhydrous conditions.
Question. Which one in the following pairs of substances undergoes SN2 substitution reaction faster and why?
Answer :

Question. Give reasons:
(i) Racemic mixture is optically inactive.
(ii) The presence of nitro group (—NO2) at o/p positions increases the reactivity of haloarenes towards nucleophilic substitution reactions.
Answer : (i) Racemic mixture contains equal amount of d and l forms, hence rotation due to one enantiomer is cancelled by another.
(ii) The presence of nitro group at o-and p-positions withdraws electrons from the benzene ring and thus, facilitates the attack of the nucleophile on haloarenes. The carbanion thus formed is further stabilised by resonance.
Question. Account for the following:
(i) The C—Cl bond length in chlorobenzene is shorter than that in CH3—Cl.
(ii) Chloroform is stored in closed dark brown bottles.
Answer : (i) C—Cl bond length in chlorobenzene is shorter than C—Cl bond length in CH3—Cl.
(ii) Chloroform when exposed to air and sunlight changes to phosgene which is a poisonous gas.
CHCl3 + 1/2 O2 → COCl2 + HCl
It is kept in dark coloured bottles to prevent the oxidation.
Question. (a) Write a chemical test to distinguish between :
(i) Chlorobenzene and benzyl chloride
(ii) Chloroform and carbon tetrachloride
(b) Why is methyl chloride hydrolysed more easily than chlorobenzene?
Answer : (a) (i) Benzyl chloride gives white precipiate with AgNO3 solution while chlorobenzene does not .
(ii) CHCl3 with aniline in presence of alc. KOH gives foul smelling isocyanides whereas CCl4 does not.
(b) CH3Cl is hydrolysed easily than C6H5Cl as chlorobenzene has partial double bond character between C—Cl bond which is dificult to break.
Question. Which will react faster in SN2 displacement, 1-bromopentane or 2-bromopentane and why?
Answer : 1-Bromopentane is a primary alkyl halide, hence reacts faster in SN2 displacement than secondary halide 2-bromopentane.
Question. (i) Which alkyl halide from the following pair is chiral and undergoes faster SN2 reaction?

(ii) Out of SN1 and SN2, which reaction occurs with
(a) inversion of configuration
(b) racemisation?
Answer :

(ii) (a) SN2 reaction occurs with inversion of configuration.
(b) SN1 reaction occurs with racemisation.
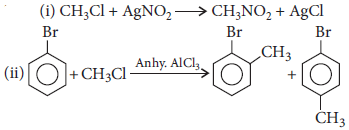
Question. Write chemical equations when
(i) methyl chloride is treated with AgNO2.
(ii) bromobenzene is treated with CH3Cl in the presence of anhydrous AlCl3.
Answer :
Question. Which will react faster in SN1 displacement reaction :
1-Bromobutane or 2-bromobutane and why?
Answer : 2-Bromobutane will react faster in SNl displacement reaction because it will form more stable secondary carbocation intermediate.
Short Answer Questions :
Question. Haloalkanes undergo nucleophilic substitution whereas haloarenes undergo electrophilic substitution. Explain.
Answer : In haloarenes –ve charge gets localised on arenes using resonance, therefore they undergo electrophilic substitution.
Haloalkanes have electrophilic carbon centre due to polarity of C→–X bond.
Question. What are ambident nucleophiles? Explain with an example.
Answer : A nucleophile which can attack from more than one centres, is known as ambident nucleophile.
e.g., -••C≡N: Cyanide ion
Question. Give reasons :
(i) C—Cl bond length in chlorobenzene is shorter than C—Cl bond length in CH3—Cl.
(ii) SN1 reactions are accompanied by racemization in optically active alkyl halides.
Answer : (i) In halobenzene C—X bond has partial double bond character due to resonance while CH3—X bond is single bond.
Thus bond length of C—X bond in halobenzene is smaller than that in CH3—X.

(iii) In SN1 reaction carbocation intermediate is formed which is a planar molecule so,an incoming nucleophile can attack from either side and a equilmolar mixture of two components are formed and resulting mixture is optically inactive.
Question. Write the balanced equation for the following:
(i) When chloroform is oxidised by air.
(ii) Chloroform reacts with chlorine.
Answer :

Question. Give reasons for the following :
(i) Ethyl iodide undergoes SN2 reaction faster than ethyl bromide.
(ii) C—X bond length in halobenzene is smaller than C—X bond length in CH3—X.
Answer : (i) Since I– is a better leaving group than Br–, thus, CH3CH2I undergoes SN2 reaction faster than CH3CH2Br.
(ii) C—Cl bond length in chlorobenzene is shorter than C—Cl bond length in CH3—Cl.
Question. Predict the order of reactivity of the following compounds in SN1 reaction.
C6H5CH2Br, C6H5C(CH3)(C6H5)Br,
C6H5CH(C6H5)Br, C6H5CH(CH3)Br
Answer : C6H5C(CH3)(C6H5)Br > C6H5CH(C6H5)Br > C6H5CH(CH3)Br > C6H5CH2Br
Question. Answer the following questions:
(i) What is meant by chirality of a compound?
Give an example.
(ii) Which one of the following compounds is more easily hydrolysed by KOH and why?
CH3CHClCH2CH3 or CH3CH2CH2Cl
(iii) Which one undergoes SN2 substitution reaction faster and why?
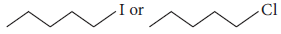
Answer : (i) Chiral object : An object which has no plane of symmetry (cannot be divided into two identical halves) is called chiral (Greek; Chiral-Hand) or dissymmetric or asymmetric. A Chiral object is not superimposable on its mirror image.
e.g., left and right hand of a person are mirror images of each other and are not superimposable.

Question. Which compound in the following couple will react faster in SN2 displacement and why?
(i) 1-Bromopentane or 2-bromopentane
(ii) 1-Bromo-2-methylbutane or 2-bromo-2-methylbutane.
Answer : (i) 1-Bromopentane, as it is a primary alkyl halide.
(ii) 1-Bromo-2-methylbutane, as it is a primary alkyl halide.
Question. (i) Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism and why?

(ii) Racemisation occurs in SN1 reactions.
Why?
Answer : (i) 1-Bromobutane is 1° alkyl halide while 2-bromobutane is 2° alkyl halide. Due to steric hindrance in 2° alkyl halides, 1° alkyl halide will react faster than 2° alkyl halide in SN2 reaction.
(ii) Carbocations are formed in SN1 reaction which are planar species, thus, racemisation occurs.
Question. Give reasons for the following observations :
(i) Haloarenes are less reactive than haloalkanes towards nucleophilic substitution reactions.
(ii) The treatment of alkyl chloride with aqueous KOH leads to the formation of alcohol but in the presence of alcoholic KOH, alkene is the major product.
Answer : (i) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions due to the following reasons.
(a) Resonance effect : In haloarenes the electron pairs on halogen atom are in conjugation with p-electrons of the ring and the following resonating structures are possible.

C—Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is dificult than haloalkane and therefore, are less reactive towards nucleophilic substitution reaction.
(b) difference in hybridisation of carbon atom in C—X bond.
(ii) In aqueous solution, KOH is almost completely involved to give OH– ion which being a better nucleophile gives a substitution reaction on alkyl halides to form alcohol. But an alcoholic solution of KOH containing alkoxide (RO–) ions which being a much stronger base than OH– ion preferentially snatches a H+ ion from an alkyl chloride to form alkenes.
Question. Explain why in the pair, (CH3)3CCl and CH3Cl will react faster in SN2 reaction with OH– ?
Answer : CH3Cl will react faster in SN2 reaction with OH–.
Question. Write the major product(s) in the following :

Answer :

Question. (i) Why is it that haloalkanes are more reactive than haloarenes towards nucleophiles.
(ii) Which one of the following reacts faster in an SN1 reaction and why?

Answer : (i) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions due to the following reasons.
(a) Resonance effect : In haloarenes the electron pairs on halogen atom are in conjugation with p-electrons of the ring and the following resonating structures are possible.

C—Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is dificult than haloalkane and therefore, are less reactive towards nucleophilic substitution reaction.
(b) difference in hybridisation of carbon atom in C—X bond.
(ii) Tertiary halide Cl reacts faster than the secondary halide because of the greater stability of tert-carbocation.
Question. Which one in the following pairs undergoes SN1 substitution reaction faster and why?
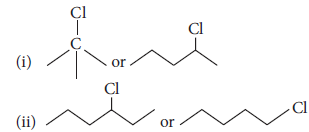
Answer : (i)

Tertiary halide reacts faster than secondary halide because of the greater stability of tert. carbocation.

Question. Why is the following occur :
Chloroform is stored in closed dark coloured bottles completely filled so that air is kept out.
Answer : Chloroform when exposed to air and sunlight changes to phosgene which is a poisonous gas.
CHCl3 + 1/2 O2 → COCl2 + HCl
It is kept in dark coloured bottles to prevent the oxidation.
Question. (i) Why are haloalkanes more reactive towards nucleophilic substitution reactions than haloarenes?
(ii) Which one of the following two substances undergoes SN1 reaction faster and why?

Answer : (i) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions due to the following reasons.
(a) Resonance effect : In haloarenes the electron pairs on halogen atom are in conjugation with p-electrons of the ring and the following resonating structures are possible.
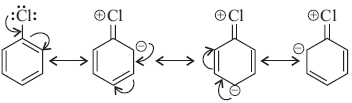
C—Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is dificult than haloalkane and therefore, are less reactive towards nucleophilic substitution reaction.
(b) difference in hybridisation of carbon atom in C—X bond.
Question. How are the following conversions carried out?
(i) Benzyl chloride to benzyl alcohol,
(ii) Methyl magnesium bromide to methylpropan-2-ol.
Answer :

Question. Give one example of each of the following reactions :
(i) Wurtz reaction
(ii) Wurtz-Fittig reaction.
Answer : (i) Wurtz reaction : It converts alkyl halide into higher alkane in presence of sodium metal and dry ether.

Question. What is Saytzeff rule? Illustrate with suitable example.
Answer : Saytzeff rule : In elimination reaction alkene having the lesser number of hydrogen on the double bonded carbon atom is formed. This generalisation is known as Saytzeff rule for example.

